Addition Reactions of Alkenes and Markovnikov's Rule
Addition Reactions of Alkenes
General form:
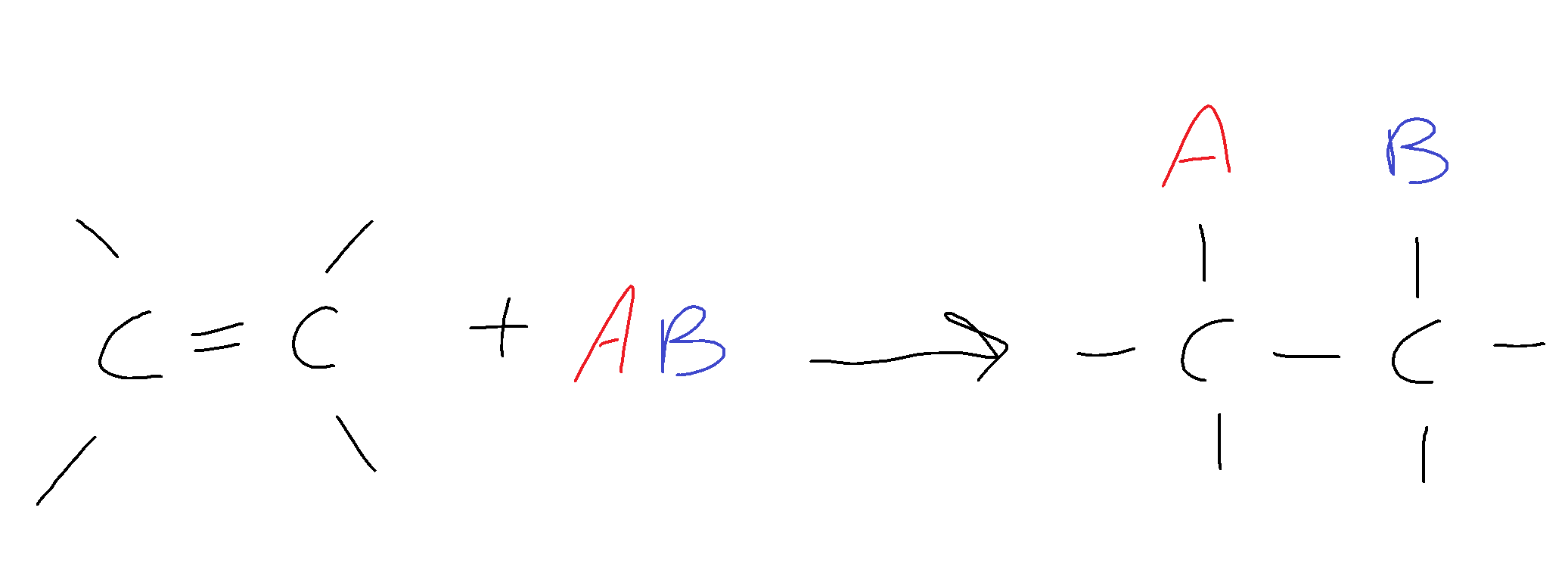
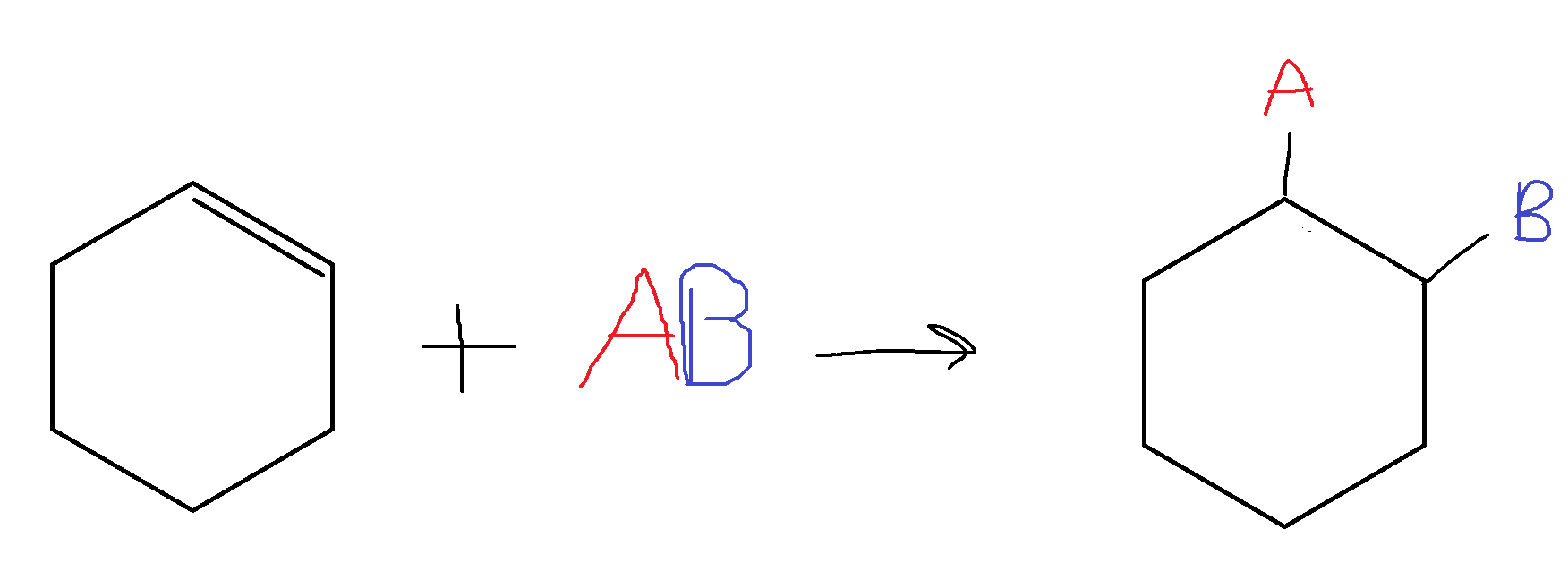
For a cyclic compound:
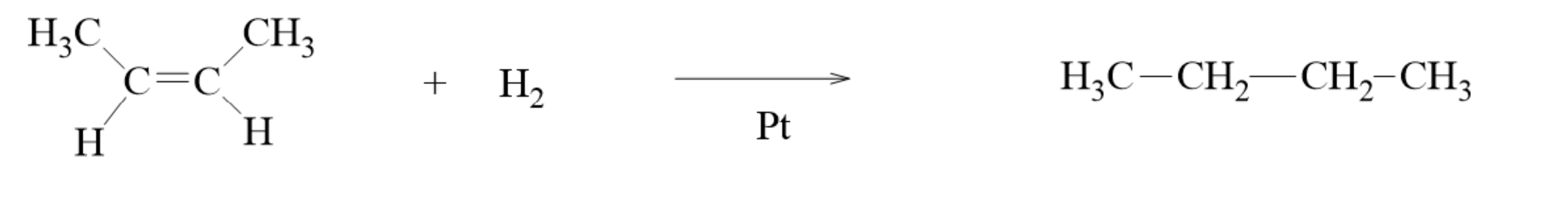
Catalytic Hydrogenation
hydrogenation- the process of adding hydrogen to carbon-carbon double bonds to make the molecule more saturated in hydrogens
This can be done by passing hydrogen gas through a solution of an alkene in the presence of a metal catalyst such as Pd, Pt, or Ni.
The product is an alkane.
having either the cis- or trans- isomer of the molecule gives you the same product
The carbons in the double bond go from sp2 hybridized to sp3 hybridized
This reaction occurs because sigma bonds tend to be stronger than pi bonds, so the pi bond in the double bond is broken easily.
This process is enthalpy favored because the alkane’s single bonds are at a lower energy level than the alkene’s double bond(s).
This process is not entropy favored because you are going from 2 reactants to 1 product.
This reaction is enthalpy driven
Addition of HCl or HBr to form a Haloalkane
The carbons in the double bond go from sp2 hybridized to sp3 hybridized
This reaction occurs because sigma bonds tend to be stronger than pi bonds, so the pi bond in the double bond is broken easily.
This process is enthalpy favored because the alkane’s single bonds are at a lower energy level than the alkene’s double bond(s).
This process is not entropy favored because you are going from 2 reactants to 1 product.
This reaction is enthalpy driven

Addition of Water to an Acid Catalyst to form an Alcohol
the catalyst is the sulfuric acid
H corresponds to A and OH corresponds to B in the general form
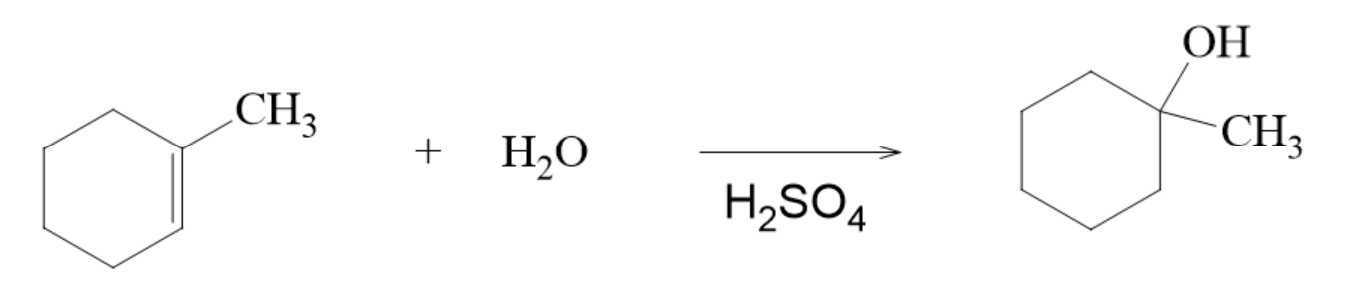
Markovnikov’s Rule
The formation of haloalkane and alcohol are regioselective reactions- meaning the reaction favors the formation/breaking of a bond at a specific location over another
- In other words- “Hydrogen goes where hydrogen is”
For example, the below reaction has two possible products:
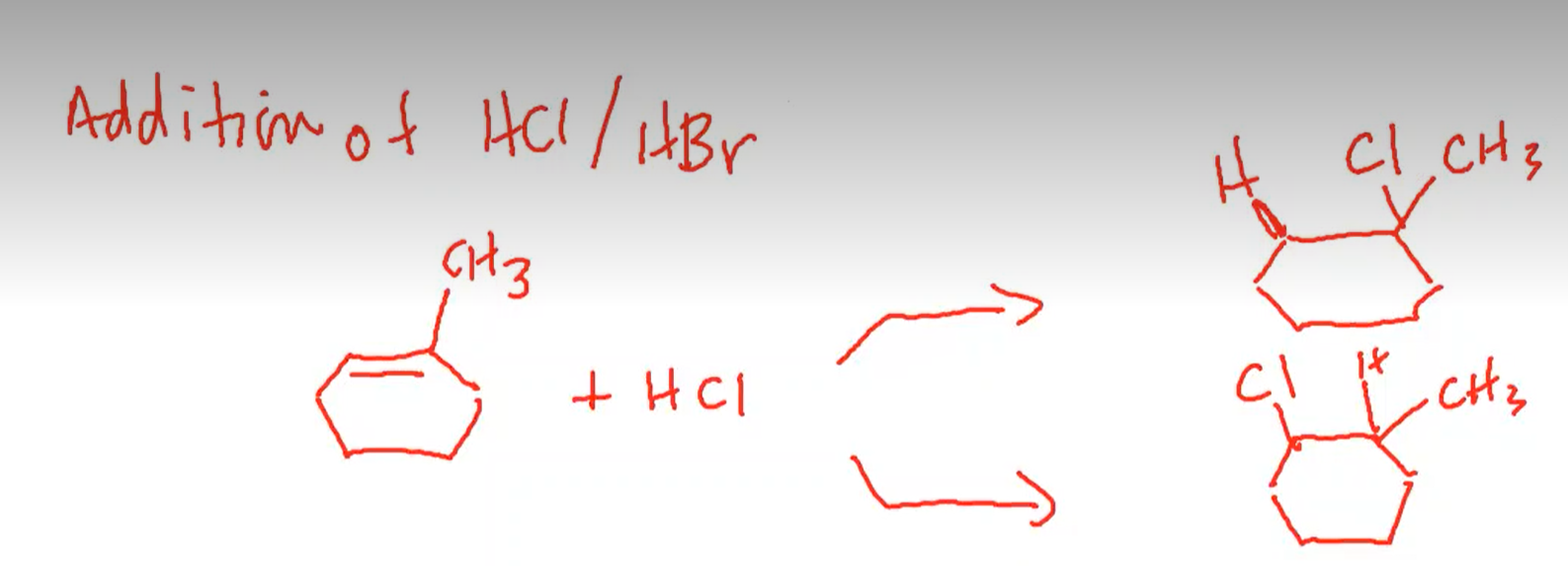
The product at the top is favored because it is more substituted
why is the more substituted product favored?- there’s a carbocation intermediate in the reaction mechanism
Carbocation Stability
Order of carbocation stability:
- tertiary carbocations- carbocations that are attached to three carbon atoms/groups
- secondary carbocations- carbocations that are attached to two carbon atoms/groups
- primary carbocations- carbocations that are attached to one carbon atom/group
- methyl carbocations- a free-standing methyl group where the carbon has a positive charge
Knowing this, we can see that the top product is favored because it allows for the formation of an intermediate tertiary carbocation that is more stable.
- because it’s more stable, this intermediate tertiary carbocation forms more rapidly
Mechanism of addition of HCl to an Alkene
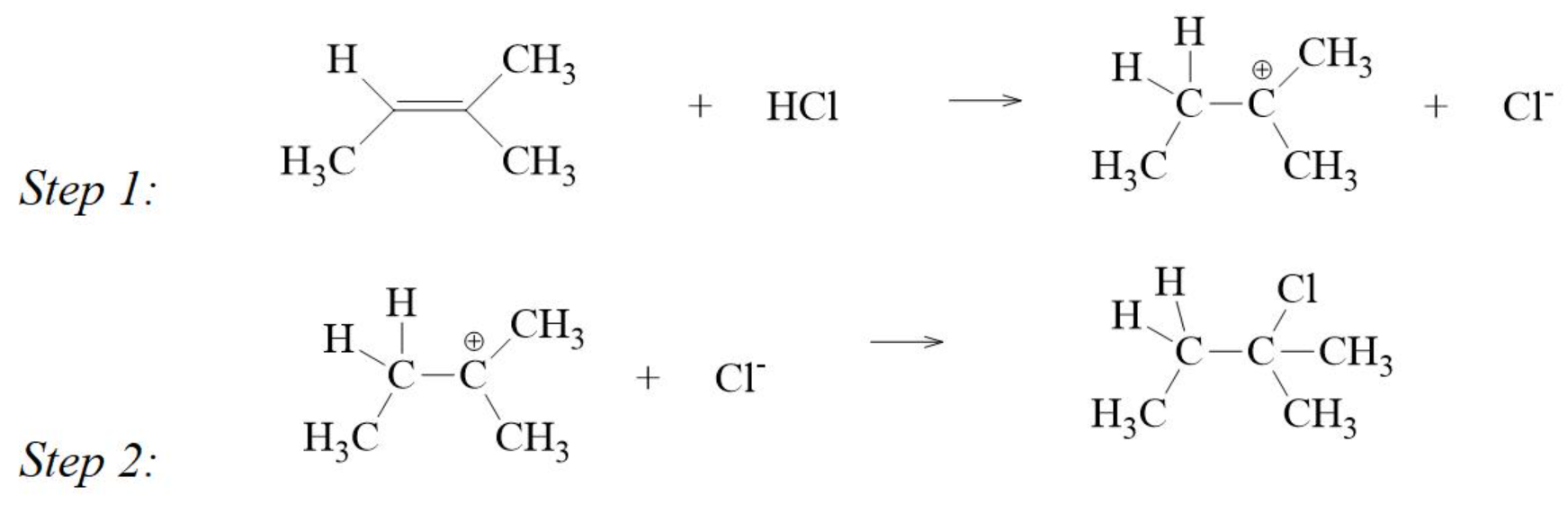
- step 1 is the slower step and determines the rate of reaction
- the addition of water to form an alcohol has a similar mechanism, and the more substituted alcohol is the major product