Lesson 7: Neurotransmitters and Their Receptors
1/56
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
57 Terms
What are the two main classes of chemical substances used as neurotransmitters in the nervous system?
Small-molecule neurotransmitters and neuroactive peptides.
What are neuroactive peptides?
Short polymers of amino acids.
How are the different types of vesicles distributed within most neurons?
Both types of vesicles (small-molecule neurotransmitter vesicles and neuroactive peptide-containing vesicles) are found in most neurons but in different proportions.
Where are both small-molecule neurotransmitters and neuroactive peptides contained?
Within vesicles.
Which type of vesicles can contain both small-molecule neurotransmitters and neuropeptides?
Large dense-core vesicles.
Where are most small-molecule neurotransmitters synthesized?
By enzymes in the cytosol.
Small-Molecule Neurotransmitters
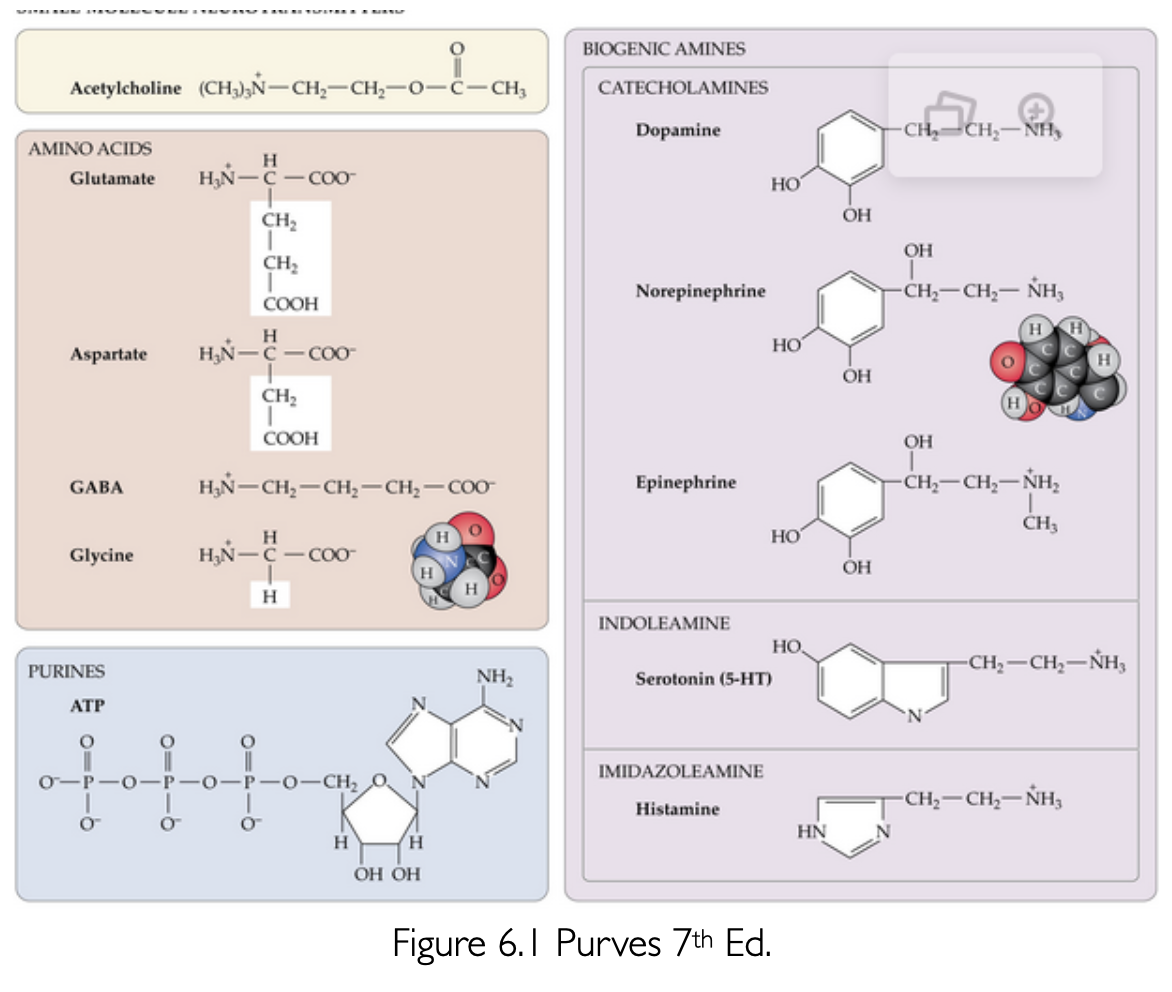
What are the typical actions of neurotransmitters, and when might they vary?
Neurotransmitters are typically either excitatory or inhibitory, but they can be both depending on the receptor they bind to.
What is required for the synthesis of a given neurotransmitter?
A precursor from which the neurotransmitter is made.
What important step exists in the synthesis of a given neurotransmitter?
There is a rate-limiting step.
Functional Features of Major Neurotransmitters
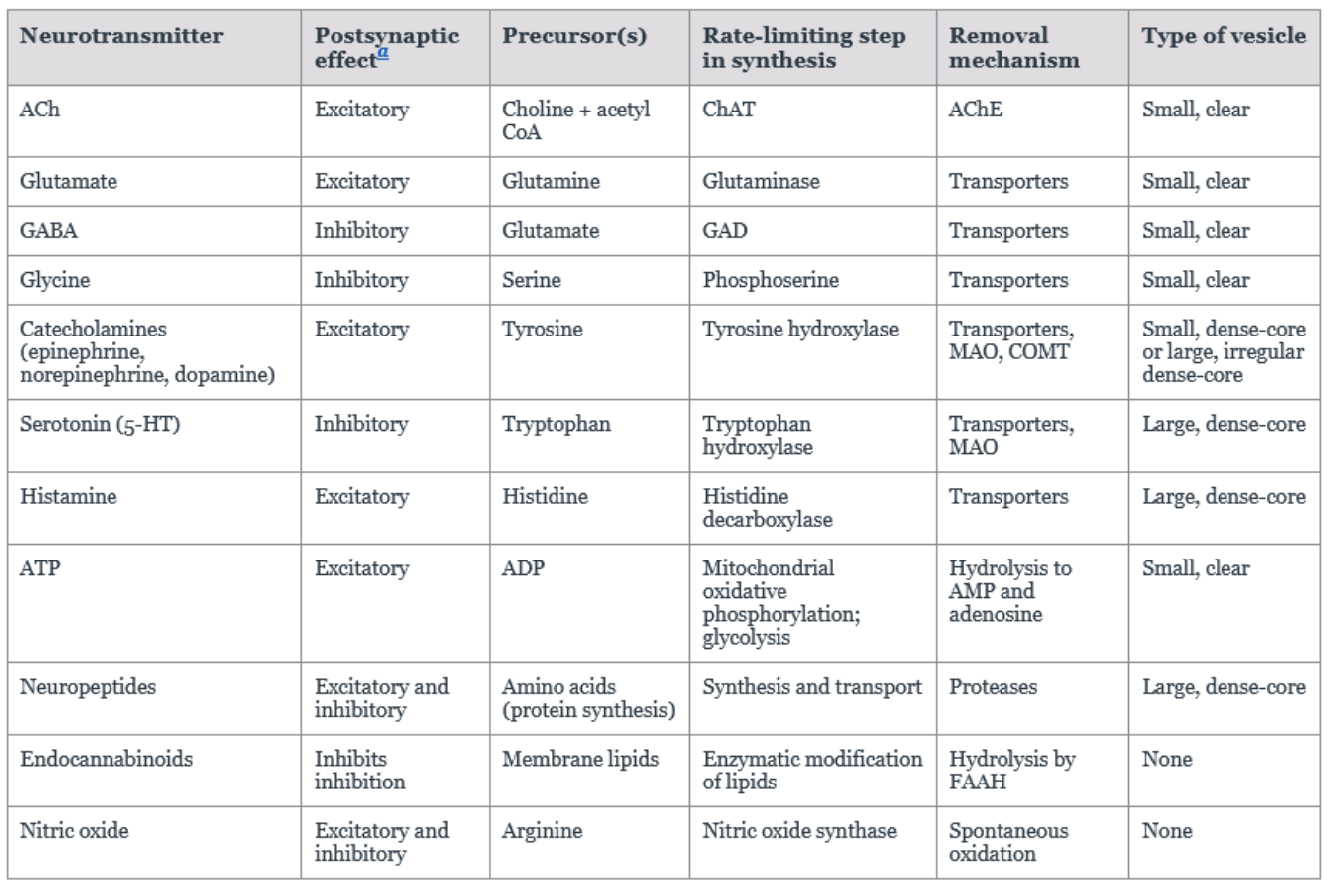
How long does the interaction between a given neurotransmitter and a receptor typically last?
Usually, it is transient, lasting only a few milliseconds to minutes.
Compare the removal speed of small-molecule neurotransmitters and larger neuropeptides from the synapse.
Small-molecule neurotransmitters are removed more quickly from the synapse than the larger neuropeptides.
How does diffusion play a role in neurotransmitter removal?
Diffusion removes some fraction of all neurotransmitters; however, glial processes covering synapses limit diffusion outside the immediate area of the synaptic cleft.
Which mechanism of degradation is used at cholinergic synapses, and what enzyme is involved?
Enzymatic degradation is used at cholinergic synapses, involving acetylcholinesterase, which hydrolyzes ACh to choline and acetate.
What is the function of other neurotransmitter degradation pathways like those involving monoamine oxidase and catechol-O-methyltransferase?
These pathways are associated with controlling neurotransmitter concentration, not with regulating synaptic transmission directly; they break down or degrade amine neurotransmitters.
What are the two primary purposes of neurotransmitter reuptake?
Reuptake serves to (1) terminate the action of the neurotransmitter and (2) recycle transmitter molecules.
What is unique about Acetylcholine (ACh) among low-molecular-weight neurotransmitters?
Acetylcholine is the only low-molecular-weight neurotransmitter that is not an amino acid or derived directly from an amino acid.
What enzyme catalyzes the synthesis of ACh (acetylcholine) from acetyl CoA and choline, and what is its significance?
it is catalyzed by choline acetyltransferase, which is the rate-limiting step.
Where does nervous tissue obtain choline for ACh synthesis?
Nervous tissue cannot synthesize choline; it is derived from the diet.
Is Acetyl CoA exclusive to cholinergic neurons?
No, Acetyl CoA is used in various metabolic pathways and is not restricted to cholinergic neurons.
Where is ACh released in the nervous system?
ACh is released at all vertebrate neuromuscular junctions (NMJs) by spinal alpha motor neurons, all autonomic preganglionic neurons, and many (not all) postganglionic neurons.
How is ACh degraded, and what happens to its metabolic products?
Acetylcholinesterase degrades ACh to acetate and choline; choline is taken up into the presynaptic terminal to be reused.
Where are cholinergic synapses found in the brain?
They are found throughout the brain, especially in the nucleus basalis (in the forebrain) and the reticular activating system (in the brain stem).
What is the primary role of Glutamate in the nervous system?
it is the major excitatory neurotransmitter.
Why must Glutamate be synthesized in neurons from local precursors?
Because glutamate does not cross the Blood-Brain Barrier (BBB).
How can glutamate be synthesized in the presynaptic terminal?
Although glutamate is synthesized from α-ketoglutarate, an intermediate in the TCA cycle, it can be synthesized in the presynaptic terminal from glutamine by glutaminase
What happens to Glutamate once it is released into the synapse?
Glutamate is taken back up by both neurons and glia.
What enzyme converts Glutamate to glutamine in glia?
glutamine synthetase (GS).
What is the fate of glutamine released by glia?
it is taken back up by neurons and converted back to Glutamate.
What kind of blocking mechanism does the NMDA receptor have?
The NMDA receptor has a voltage-dependent blocking mechanism.
What causes the voltage-dependent block of the NMDA receptor pore?
The voltage-dependent block of the NMDA receptor pore is due to Mg^{2+}.
What occurs at hyperpolarized potentials regarding the NMDA receptor pore?
At hyperpolarized potentials, the pore is blocked by Mg^{2+}.
How does depolarization affect the Mg^{2+} block of the NMDA receptor pore?
Depolarization pushes Mg^{2+} out of the pore, removing the block.
Why are AMPA receptors considered the primary mediators of excitatory transmission in the brain?
Because EPSPs produced by NMDA receptors are slower and longer-lasting than those produced by AMPA receptors.
Where is GABA present in the nervous system, and what is its primary role?
GABA is present throughout much of the nervous system and is a major inhibitory neurotransmitter.
From what precursor(s) is GABA synthesized, and by what enzyme?
GABA is primarily synthesized from glutamate the major precursor (though pyruvate and glutamine can also serve as precursors) by glutamic acid decarboxylase (GAD).
What cofactor does glutamic acid decarboxylase (GAD) require for GABA synthesis?
GAD requires the cofactor pyridoxal phosphate, which is derived from vitamin B_6.
What is the significance of a vitamin B_6 deficiency relating to GABA synthesis?
A deficiency of vitamin B_6 can lead to a decrease in GABA synthesis, which was tragically highlighted by infant deaths due to its omission in infant formula.
How is GABA transported into synaptic vesicles after synthesis?
Once synthesized, GABA is transported into synaptic vesicles by the vesicular inhibitory amino acid transporter (VIAAT).
How is GABA metabolized and what is the end product?
Most GABA is broken down (converted) to succinate, which can be metabolized further in the TCA cycle (γ-hydroxybutyrate is a GABA derivative!)
What is Glycine's primary role as a neurotransmitter, and how is it synthesized?
Glycine is an inhibitory neurotransmitter that can be synthesized by several metabolic pathways.
How does the distribution of Glycine in the CNS compare to that of GABA?
The distribution of glycine within the CNS is more restricted than that of GABA.
From what precursor is Glycine typically synthesized?
Glycine is typically synthesized from serine, which is derived from glucose.
How is Glycine transported into synaptic vesicles after synthesis?
Once glycine is synthesized, it is transported into synaptic vesicles by the vesicular inhibitory amino acid transporter (VIAAT).
What amino acid serves as the basis for the synthesis of dopamine, norepinephrine, and epinephrine?
Tyrosine.
Why are dopamine, norepinephrine, and epinephrine known as catecholamines?
Due to the benzene-OH and amine groups they possess.
What is the rate-limiting step in the biosynthesis of catecholamines, and what enzyme is involved?
it is the first step, and the enzyme tyrosine hydroxylase, converts tyrosine to L-DOPA.
How are catecholamine neurotransmitters primarily inactivated?
Largely by reuptake mechanisms, with enzymatic degradation playing a lesser role.
How many peptides act within nerve cells?
Numerous peptides act within nerve cells.
Do some peptides act as hormones outside the brain?
Yes, some peptides act as hormones outside the brain.
What is an additional source or function of some peptides related to bodily secretion?
Some are products of neuroendocrine secretion.
In what specific processes have neuroactive peptides been implicated?
Neuroactive peptides have been implicated in modulating sensory perception and emotions.