Lab #5 | Reductive Amination
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
Hydrogen
An element with one proton and one electron
Proton
Hydrogen atoms bonded to electron withdrawing groups like electronegative atoms
basically losses its one electron, meaning only one proton remains
enhanced acidity
ex: H in OH is a proton
Hydride
Hydrogens that have 2 electrons
net negative charge
hydrogens directly bonded to metals are hydrides
nucleophilic as they have a surplus of electrons
when drawn in a mechanism, arrow comes from the bond, not the hydride as all electrons are apart of the bond
What is the purpose of saving amine for TLC?
Determine reaction progress
To compare the Rf of the starting and the product
Gas is formed when quenching unreacted borohydride. What is this?
Hydrogen gas
What is the aqueous layer?
Water and byproducts from NaBH4
What is the purpose of brine?
It removed water from the organic layer
Do the hydrides in NaBH4 have lone pairs?
No, as the two electrons are confined to the metal hydride bond
SO: DRAW MECH with electrons coming from the bond
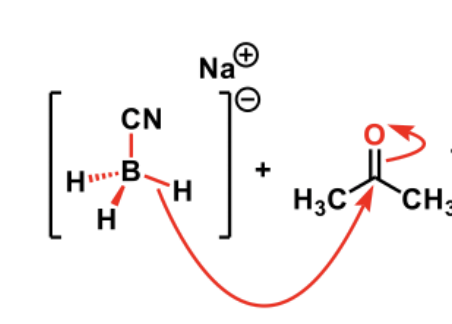
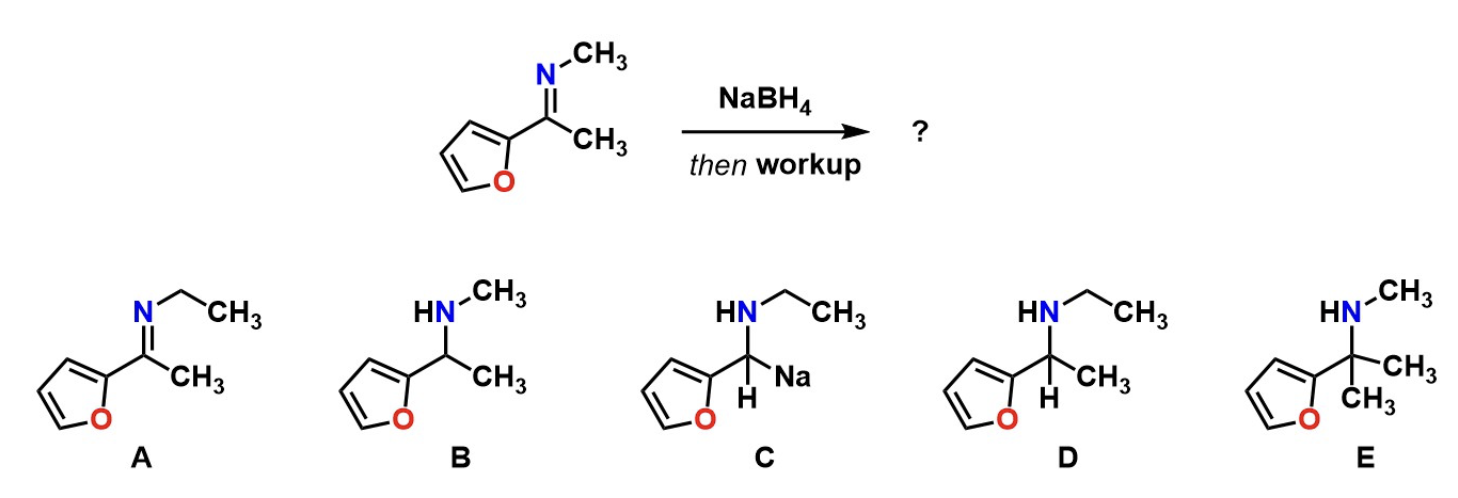
Predict product
B
Role of THF
Stabilizing solvent
Role of NaBH4
Reducing agent that adds a hydrogen
A gas is formed in quenching the unreached sodium borohydride with NaHCO3. What is this gas?
Hydrogen gas
What is the aqueous layer in this experiment?
Water and byproducts from NaBH4