Test Block One
Chapter 1: Cell Pathology
Define the two major types of cellular injury
Cell injury occurs if we go too far out of homeostasis, for too long.
Reversible Cell Injury
Only reversible if stimulus is removed, usually the injury is mild and short lived.
Example: Hydropic changes (cell swells)
Irreversible Cell Injury
REMINDER A REVERSIBLE CELL INJURY CAN CROSS THE POINT OF NO RETURN AND BECOME IRREVERSIBLE IF STIMULUS IS NOT REMOVED.
Things that can cause irreversible cell injuries: high dose heavy metals, anoxia, severe or prolonged hypoxia, etc.
Describe the cellular alteration in reversible and irreversible cellular injury
Reversible
Example: Hydrophic change (influx of water into cytoplasm)
No energy, no Na+/K+ ATPase activity, Na+ which is found in high levels outside of the cell SPRINTS down its concentration gradient (into the cell), water then chases the salt. Bada-bing Bada-boom cell swells
Decreases Energy Production
When the cell swells, the mitochondria also swells (not a good thing for the power house)
The mitochondria is less efficient when it swells and must use anaerobic glycolysis which generates less ATP
this also increases the amount of lactic acid in a cell
Decrease Protein Synthesis and Enzyme activity
Since there is more lactic acid in the cell, the pH drops.
The acidic levels of the cytoplasm decreases metabolism (enzymes can’t work in these conditions, they’re gerber daisies)
The acidic levels of the cytoplasm also starts to “beat up” the RER so protein synthesis decreases
Increase Auto-phagocytosis (eating your own self)
As damage occurs from the low pH, the cell does what it normally does - destroys them in a lysosome. These destructive elements may leak into the cell and the vicious cycle continues
Irreversible
Irreversible Injuries to the Nucleus
Pyknosis - condensation of chromatin
Karyorrhexis - nucleus fragments into smaller
Karyolysis - enzyme dissolved nucleus chromatin (DNA gone)
Describe the causes of cellular injury
Hypoxia and Anoxia
Hypoxia is low O2
Anoxia is NO O2
Most important and common type of cell injury
Cells can only survive on anaerobic respiration for so long.
Different cells can be survive without O2 for longer periods of time (they’re less sensitive to hypoxia/anoxia)
Brain cells can survive for a few min
Heart cells for a like 1-2 hours
Kidney cells for a few hours
Connective tissue cells can even last for 24 hours after death
Toxin Cell injury
Direct toxin: adverse response is directly caused by the substance, it doesn’t need to be “activated” by the body
Think heavy metals like mercury
Indirect toxin - adverse response is due to resulting metabolites
CCl4 is converted by the liver to CCl3
CCl3 is what is toxic (its a free radical)
Dose-Dependent Toxicity: Some medications can also be toxic if taken in large amounts
Tylenol is chill unless it is taken in large doses
its metabolized by the liver and in high doses the end product is toxic to the liver
Microbial Pathogens
Bacteria produce toxics that inhibit some cell functions
Food Poisoning: (salmonella/e.coli) unfrigerated leftover food is caused by exotoxins which are released by bacteria
Symptoms are a consequence of “cell poisoning”
Virus
typically “kill from within” by disturbing cellular processes or integrity of cell membrane
Can also hijack the cells machinery by sticking their DNA into our DNA
Immune system will recognize the foreign viral proteins and attack the cell
Genetic and Metabolic Disturbances
Many genetic diseases adversely affect the normal intermediate metabolism with subsequent accumulation of toxic metabolites in the cells
Example
DM
hyperglycemia which alters the metabolism of major organs, such as the liver or kidneys
Also produces pathologic changes in small blood vessels which will impede mircocirculation and cause pathologic tissue changes related to chronic hypoxia
Explain cellular adaptation to injury
Prolonged exposure of cells to adverse or exaggerated normal stimuli leads to adaptation of the cells
Atrophy: cell/tissue/organ/entire body gets small or reduced number of cells/tissue/organ/entire body
Physiologic atrophy occurs with age and involves the entire body basically
Pathologic atrophy typically occurs as a result inadequate nutrition, oxygen supply, or hormonal stimulation
General body wasting from cancer/malnutrition
Damaged or old organelles are taken up by autophagosomes and degraded
Undigested residues can be seen in the cytoplams as lipofuscin which is what makes the cadavers brownish
Extra proteins released from damaged organelles are mark for disruption by ubiquitin a scavenger protein
Hypertrophy and Hyperplasia
Hypertrophy: an increase in the size of tissue or organs caused by enlargement of individual cells
Hyperplasia: an increase in size of tissue or organs caused by a an increase in the number of cells
Hypertrophy and hyperplasia are besties, they usually go everywhere together
Hypertrophy occurs by itself only in the heart because these cells cannot divide (AKA Pure hypertrophy)
Hypertrophy with hyperplasia occurs in lots of conditions like when the bladder wall thickens when obstructed by BPH or in uterine smooth muscle cells in pregnancy
Physiologic Hypertrophy: skeletal muscles get swoll when you work out
Pathological Hypertrophy: heart muscle in CHF
Pure hyperplasia typically results from hormonal stimulation
Endometrial hyperplasia with estrogen (may progress to neoplasm)
BPH
In chronic injury, hyperplasia may also occur (like you wear high heels everyday)
Hyperplastic lesions (like polyps) may have no obvious cause (idiopathic) and are probably some early neoplasms
Metaplasia (Metamorphosis)
Changing from one cell type to another
In smoking, the ciliated columnar bronchial epithelium into squamous epithelium
Can be reversible - like if you stop smoking - but if stimulus remains it progress to dysplasia (disorderly arrangement of cells) which may progress to neoplasm.
Intracellular Accumulations
Intracellular accumulation may result from an overload of various metabolites or exogenous materials
exogenous examples
Example: Anthracosis (Coal Lung)
Coal particles get stuck in the lungs
Endogenous Examples:
Hemosiderosis: an accumulations of blood-derived brown pigment (hemosiderin) which is usually derived from hemolyzed RBCs.
Its just the aggregation of ferritin
Prussian Blue Stain
Can occur in the livers of people who get lots of blood transfusions and in those with hemolytic anemia
Can also result as a genetic disorder where you cannot absorb iron in food
Lipid Accumulation
Can occur in the liver (like fatty liver cirrhosis) from deposit of triglycerides
Define the two types of cellular death
Reminder: necrosis and apoptosis happen to cells in LIVING individuals
If the person is dead, then it is known as autolysis
Necrosis: exogenously induced cell death
tends to be messier (cell swells and ruptures, membrane is destroyed)
when the cell explodes it will affect multiple cells
used in immune functions
vital processes of the cell are inhibited
Ends with phagocytosis of NEUTROPHILS (polymorphonuclear)
Apoptosis: endogenously programmed cell death (may also be exogenous)
This process is energy dependent and vital processes maintained
AKA active cell death
tends to be cleaner and only affect one cell
The cell membrane is fully intact but, the insides are fragmented (apoptotic bodies)
Ends with phagocytosis of macrophages
suicide genes
Physiological: In fetal development the cells that make of the webbing of the fingers die or clonal deletion in the immune system or cells that are just not needed anymore
Pathological: Cells with DNA damage/ER stress or infections
Lack of apoptosis is also a problem, like if the fetus’ finger webs don’t die we get syndactyly or in some cancers (like follicular lymphoma (NHL)) cells forget to die
Describe the various types of necrosis
Secondary liquefaction necrosis AKA Wet gangrene may happen after coagulative necrosis
the dead tissue gets infected by the bacteria, you get inflammation and it smells like death
Very common in the feet of DM patients
If necrotic tissue dries out like The Mummy (with academy award winning Brendon Fraiser) its Dry gangrene
Usually due to a lack of blood-flow like frost bite
Necrotic tissue attracts calcium salts and often calcifies (duh)
This is dystrophic calcification
Fun fact: maggots are used to treat patients with necrosis that aren’t candidates for surgery because maggots only eat necrotic tissue
Gas gangrene is a medical emergency that usually results in deep trauma wounds (combat injuries/surgical settings)
Bacteria gets in there, releases toxins, blood flow is disrupted
you get bubbles and crepitus (snap, crackle, pop)
Smells like death
Blisters usually drain
Coagulative Necrosis
most common form of necrosis
Marked by rapid inactivation of cytoplasmic enzymes so lysis is inhibit and tissues maintain their form and consistency
Proteins are denature and it “gunks" up the works
Very common in solid organs (heart, liver, kidneys)
Liquefactive Necrosis
Marked by rapid liquefaction by enzymes that results in abscess or pus formation
Pus tends to be full of dead and dying leukocytes and debris
Most often in soft/fatty tissue (like the brain)
Caseous Necrosis
Typically found in TB patients or fungal infections
The love child between liquefactive and coagulative
Tissue is “cheesy” because it is destroyed from the inside out
Enzymatic Fat Necrosis
Special type of liquefactive that is caused by lipolytic enzymes
Marked by chalky white/yellow deposits (soap scatter)
its literally soap, the enzymes melt the fat and it binds to calcium (you’ve seen fight club you know how this works)
usually seen in fatty tissues like pancreas and breast
In pancreatitis, this can be seen on CT and is know as stratification
Chapter 2: Inflammation
List the signs of Inflammation
First described by our Roman homie Celsus
Calor
Heat
Rubor
Redness
Tumor
Swelling
Dolor
pain
Loss of Function
the latin name is functio laesa if you wanna be fancy
Explain the inflammatory process
Circulatory Changes
Changes in blood flow are the bodies 1st response to injury (vascular spasm or vasoconstriction)
Vasoconstriction only last for a few seconds
Next we get vasodilation, the capillaries are quickly filled with blood
hydrostatic pressure increases, filtration increases, we get light swelling
More blood in the capillaries also causes the red appearance
Since injured tissues need more blood, we get active hyperemia
We know what it is but the patho book describes it as an influx of blood into inflamed areas
Vascular Changes
Most of the changes have to do with
increase hydrostatic pressure
slowing down of circulation
adhesion of leukocytes and platelets
Release of soluble mediators from WBCs, platelets, and endothelial cells
Humeral Response
The release and action of soluble mediators produced by inglammatory cells and various organs in responds to injury (see chemical mediators)
Cellular Response
Blood flow in the capillaries is slow (greater cross section area) this leads to redistribution of RBCs and WBCs
RBCs form stacks (rouleaux) which impede circulation and lead to turbulent flow
WBCs undergo Margination (pushed to the walls of the capillaries)
pavementing is them attaching to the walls
As leukocytes activate, the develop long protrusions which allow for them to stick better to the endothelial walls
in neutrophils the adhesion molecules are selectin and integrin
these molecules are only found on ACTIVE leukocytes
the molecules themselves are activated by cytokines (IL or TNF which are found in high concentration at sites of inflammation)
Increase permeability of vasculature last for a little while, more and more fluid leaks into the interstitial space aka Transudation
Pure fluid is transudate
As cells start to migrate into the interstitial space, we get exudate (exudate = presence of cells) which is more protein rich
Usually the cells in exudate are PMNs (polymophonuclear nuetrophils)
Inflammation begins when PMNs get into the tissue (Here’s the order of actions)
adhesion to endothelial cells
insertion of cytoplasmic pseudopods between the junctions of endothelial cells.
passage through basement membrane
amoeboid movement away from vessel toward inflammatory site (Chemotaxis)
chemoattractants mediate chemotaxis and are at the highest concentration at the site of inflammation
RBCs do NOT usually migrate into the tissue but if the space is big enough and they leak through then its call diapedesis
When PMNs get to the inflammatory site, phagocytosis commences
mediated by opsonins (from complement or antibodies)
Bacteria or whatever is engulfed and is then killed by bactericidal substances like hydrogen peroxide or free radials
the killing occurs when lysosomes fuse with the phagocytic vacuole
PMNs usually die in the process of fighting the bacteria (fallen soldiers), this is what is found in pus
inflammation dominated by pus = purulent inflammation
Discuss the cells involved in the inflammation
PMNs (Neutrophils)
must abundant WBC (60%-70%)
segmented nucleus with granules in the cytoplasm
Most important features
mobility
first to show up to the inflammation party
Phagocytosis
Bactericidal activity
granules contain hydrogen peroxide and free radicals that will beat up the bacteria
Cytokine production
secrete more inflammatory mediators like IL-1 which is an endogenous pyrogen (causes fevers)
Eosinophils
Make up 2-3% of circulating WBCs
Usually show up a few days after the neutrophils (slower motility)
Still mobile, phagocytic, and bactericidal
Interact with basophils and are prominent in type 1 hypersensitivity reactions
Parasitic infections
long living so may be seen in chronic inflammation
Basophils
Make up less than 1% of circulating WBCs
Most prominent in type 1 hypersensitivity reactions
Precursors of mast cells which are tissue-based basophils
Macrophages
tissue based derived from blood monocytes
Appear at inflammation sites 3-4 days
Phagocytes and active in bacterial killing, but their not as efficient as PMNs
Produce cytokines that activate healer cells like myofibroblast, angioblast, fibroblast
Platelets
fragments of the cytoplasm released from megakaryocytes
No nucleus but they do have granules that contain various chemicals like (histamine, cytokines, coagulation proteins, growth factors (PDGF))
granules are released when platelets make contact with the extracellular membrane
PDGF promotes proliferation of connective tissue cells
Other
Lymphocytes and plasma cells are components of chronic inflammation
Fibroblast and angioblast participate in chronic inflammation and in healing
Describe the chemical mediators involved in inflammation
Chemical mediators of the vascular changes can be put into 2 categories
Plasma Derived which circulate in an inactive form and must be activated
Cell-derived which are stored in granules of platelets or leukocytes, however they may be made on demand (De novo)
Histamine is preformed which is why it works fast
Prostaglandins have to be made from arachidonic acid which takes time
Mediators are multifunctional and had numerous effects on blood vessels but just think vasodilation, constriction, vascular permeability, activation of immune cells, chemotaxis, etc.
Typically biogenic amines, proteins, or lipids
Histamine
Bioamine stored in granules of platelets, basophils, and mast cells
Acts on endothelial cells of the venules
increase vascular permeability
increase filtration → edema
Inactivated by hisaminase pretty quickly so it is called an immediate transient reaction
Bradykinin
Kinda like histamine but acts slower
Activated in the plasma by the enzyme kallikrein which is activated by coagulation factor XII (AKA Hageman’s factor)
Hageman’s factor activates both complement, clotting, fibrinolysis, and chemotaxis
Incites pain (the dolor of inflammation)
Complement proteins
A Cascade in which the proteins are numbered C1-C9 and there are 3 ways to activate the cascade which all end with the MAC (membrane attack complex)
The MAC is an enzymatically active complex that bores holes into cell membrane
The cleaved activated complements C3a and C3b are active components as well
C3b acts as a an opsonin
C3a acts as an anaphyloxins which cause vasodilation, increase vascular permeability, and promote chemotaxis
The complement proteins are constantly floating around and are activated under the right conditions
Classical pathway
Typically activated by an antigen-antibody complex (can also be activated by C1)
Madi’s extra information for completion: macrophages make contact with a bug and produce interleukin 6, this acts on the liver to produce C-reactive protein (a pentamer). This C reactive protein binds to the surface of a pathogen and acts as a landing zone for the C1 complement protein. C1 (or an antibody) binds C4 and C2 which is cleaved to C4b and C2a respectively these combine to form C4b2a AKA the the classical C3 convertase. C3 is cleaved to C3a (anaphatoxin) and C3b which combines with the classical convertase forming the Classic C5 Convertase which can actually get us to the MAC
Alternative Pathway
Named because it does not have anything with immune reactions and is activated by bacterial endotoxins, fungi, snake venom etc.
Madi’s extra info: Starts with C3 which is activated by water into iC3, which you would think is a problem except water is found in high concentrations at microbe surface. iC3 which binds B, which binds D (a protease) D cleaves B and forms the soluble C3 convertase this chops up a whole bunch of C complement proteins into C3a (anaphylatoxins) and C3b which binds to the pathogen surface. So follow the same steps that we used to form the soluble C3 convertase but this time we’re attached to the microbe forming the alternative C3 convertase. When this binds an extra C3b we form the C-5 convertase which can get us to the MAC
Lectin Pathway
Activated by the binding of plasma mannose-binding lectin to surface carbohydrate on bacteria
Madi’s Extra info: The liver produces mannose binding lectin when macrophages release IL-6. This protein binds C4 and cleaves it to C4a (anaphlaxin) and C4b binds the surface. The mannose cleaves C2 to C2a which binds the to C4b forming the classical C3-convertase just like in the classical
Arachidonic acid derivatives
Derived from phospholipids of cell membranes through the action of phospholipases
Lipoxygenase pathway
leads to the formation of leukotrienes (LTs) these promote chemotaxis and increase vascular permeability, bronchospasm
typically seen in anaphylactic shock
Lipoxins inhibit chemotaxis and serve as the negative regulators of leukotrienes as well as act in vasodilation, inhibition of neutrophil chemotaxis, monocyte adhesion
Cyclooxygenase pathway (COX)
Prostaglandins (PGs) and thromboxane
Prostaglandins cause vasodilation, increase vascular permeability, mediate pain, and fever.
Prostacyclin (PGI2) counteracts thromboxane
Thromboxane promotes platelet aggregation, thrombrosis, and vasoconstriction
The Arachidonic acid pathways can be inhibited at many spots
Corticosteroids act on phospholipase which is involved in generating the arachidonic acid (knocks out lipoxygenase and cyclogenase)
Aspirin knocks out the COX pathways
Can be used for treatment of chronic inflammatory disease like RA and asthma
Define how inflammation is classified
Duration
Acute inflammation is usually sudden onset and last from a few hours to a few days
like a cold
Chronic inflammation last longer usually weeks to months but can even be years
Usually related to acute and is a result of the following events
Extension of acute inflammation
prolonged healing of acute inflammation
persistence of causative agents
Primary chronic inflammation evolve without a typical acute phase
Secondary chronic inflammation is preceded by an acute phase
Can also develop as a response to foreign bodies like in chronic lung solicosis
Etiology (AKA what causes it)
infections
you know the vibes (bacteria, fungi, virus, etc)
chemical causes
Organic/inorganic, industrial/medicinal, exogenous/endogenous
Physical causes
trauma, heat, radiation
foreign bodies
like in sutures or thorns
immune causes
typically related to hypersensitivity reactions
Location
Localized
widespread
Bacteremia in the blood → septic shock (FULL BODY)
Pathological features
Several forms can be seen with the human eye like changes in skin, eyes, oral mucosa, genitals
Or even in surgery
Explain the various pathologic forms of inflammation
Note: if it ends with -itis its inflammation of whatever the thing is
Serous inflammation
Characterized by exudate in serum
Occurs in most early stages of inflammation
Ex: Skin vesicles in herpes, second degree burn blisters
The Peritoneum, pleura, and pericardium can also have serous inflammation which are all characterized by accumulation of clear, yellowish fluid in the cavities
You can get it in the joints like in trauma or RA
Fibrinous Inflammation
Characterized by an exudate RICH in fibrin (plasma protein)
extravasation of fibrin only results through large spaces in the vasculature so the inflammation was BAD
Ex: strep throat, bacterial pericarditis
surface is covered in shaggy, yellow layers of fibrin
Doesn’t resolve as easily
Purulent inflammation
Typically caused by pus forming bacteria (strep or staph)
Reminder: Pus is full of dead/dying PMNs and necrotic tissue debris
If there is fibrin in the pus, we can call it fibrinopulent
Abscess are an example of this where the pus accumulates in the newly formed tissue space
Lance and drain that ho
If Large abscess rupture it forms a sinus (like popping a pimple and the pus hits the mirror that you just cleaned (open to the world)) or a fistula (a channel forms between 2 pre-existing cavities)
Empyema is accumulation of pus in a pre-exsiting cavity
Ulcerative inflammation
Characterized by formation of an ulcer of the skin/mucosa
Reminder: ulcer is an defect involving the epithelium but it may extend into the deep connective tissue
Super common in the stomach or duodenum
Psudeomembranous inflammation
A special type of ulcerative inflammation that combines with fibrinopulent exudation
Ex: C-diff secretes exotoxins that kill intestinal cells leading to ulcers and exudations in the form of pseudomembranes
Ex: Diphtheria
Chronic Inflammation
We’ve been like this (it last a long time)
Produces more extensive tissue destruction, heals less readily, and is associated with more serious function loss
Marked by an exudate full of lymphocytes, macrophages, and plasma cells
usually accompanied by scarring
Chronic pelvic inflammatory disease scars fallopian tubes
Fibrosis may also occur
Granulomatous Inflammation
Wouldn’t you know, granulomatous inflammation is characterized by granulomas
Granulomas are formed by Ts, macrophages, and multi-nucleated giant cells
Ex: TB, sarcoidosis
May be caused by antigens that cause Type IV hypersensitivity reaction
Cytokines produced by T cells transform macrophages to epitheliloid cells which combine to form multi-nucleated giant cells
Often associated with caseous necrosis
Chapter 3: Immunopathology
Describe the types of immune response
Innate Immunity (primitive and nonspecific)
This is what you’re born with
Not dependent on exposure
Includes Defense Mechanism such as:
Mechanical Barriers (skin)
Intact skin is probably the best defense
1st line of defense
Cellular responses (PMNs, phagocytes, macrophages)
Protective proteins (complement, lysosines)
Includes the 1st and 2nd lines of defense
Second line of defense is the inflammatory response and phagocytosis
Acquired Immunity (AKA adaptive immunity)
based on the ability to determine self vs. nonself
Immunocompetence: the whole system is working in unison
Opposite of immunodeficient
Basically just the B and T cells response
ANTIGEN SPECIFIC
3rd line of defense
these only engage as a last resort, ie. the other two lines have fallen
Discuss the cells of the immune system and antibodies
Lymphocytes
T Cells
CD4 or helper Ts activate macrophages and other cells to do their job better
CD8+ or cytotoxic Ts intend to kill any cell infected with microbes or cancer once they are activated
T cell receptors (CD8 or CD4) need their antigen presented in MHC (AKA human leukocyte antigen (HLA)).
MHC type 1 interact with CD8
MHC type 2 interact with CD4
B Cells
Plasma cells - aka antibody factories
5 classes of immunoglobulins (just bound antibodies)
IgM: neutralizes microorganisms, strong complement activator, and usually bind BLOOD GROUP ANTIGENS
IgG: acts as an opsonin (seasoning for phagocyte)
IgE: mediates hypersensitivity type I reactions or parasite combat (if you see IgE think basophil or mast cells)
IgA: protection of mucosal surfaces, this one is found in our secretions
IgD: involved in the antigen activation of B cells
Basically all immunoglobulin start as IgD and then differentiate
Antibodies are all composed of heavy and light chains
each chain has a constant region and a variable region
Variable region binds antigen
Light chains are either Kappa or lambda (not really important)
Heavy chains are what make the Ig different
Memory B cells - make the response faster next time
Natural Killer cells
non-specific, we’re here to kill
Macrophages
Phagocytes seen in the acute/immediate reaction
reminded a lot of these cells are primarily found in lymph tissues which is where the immune response typically begins - stay ready so we don’t have to get ready
Describe the 4 major types of hypersensitivity
Type I Anaphylactic
The only TRUE allergic reaction mediated by IgE
After the first exposure, Mast cells get sensitized and cover themselves in IgE, so on the second exposure when IgE grabs the allergen the mast cell degranulates and releases histamine
Histamine can go systemic and act on blood vessels resulting in acute edema
Where ever the histamine response occurs is where the system is affected
typically atopic in nature
immediate response in minutes
latent response some time after that
Examples:
Hay Fever (allergic rhinitis)
Asthma
Atopic dermatitis
Anaphylactic shock
if you wanna be fancy this is a low resistance shock because histamine is a vasodilator
Medical Emergency
it’s EPI TIME BABY
Type II Cytotoxic Antibody Mediated
Typically mediated by IgG
Cells are either killed through lysis via MAC (IgG activates complement) or cytotoxicity (killed by CD8s or NKs)
Disease Examples:
Hemolytic Anemia
RBCs get lysed and the bone marrow can’t keep up
Lab values are gonna show a high reticulocyte count
Symptoms: heart palpitations, palor, SOB, hepatosplenomegaly, fever, abdominal/back pain, bad cases may go into shock
Goodpasture’s syndrome
Affects the kidneys
form of glomerulonephritis
rapid progression
pretty rare more common in young men
IgG is deposited in kidneys or lungs
Symptoms: edema, dysuria, HTN,
Labs: bolod in urine, RBC cast, protein in urine
Treatment: high dose steroids, plasmapheresis, ace inhibitor
Grave’s disease
Affects the thyroid
Over stimulation of the thyroid (antibodies bind TSH receptors)
Symptoms: Bug eyes, pretibial myxedma,
Testing: radioactive iodine
Treatment: thyroidectomy or antithyroid meds
Myasthenia Gravis
Neuromuscular junctions (ACh receptors)
typical patient population: Women 20-40
symptoms: episodic weakness, easy muscle fragility, ptosis, respiratory compromise
Type III Immune Complex Mediated
Antibody complexes are put where they aren’t supposed to be, these lead to complement activation and leukocyte response
Disease Examples:
Systemic Lupus Erythematous (SLE)
affects multiple systems antibodies against our own nuclei
Common in African American women
infections, nephritis, and CNS infections occur
Labs to run: ANA (antinucleated antibody) or a biopses
blood in urine, elevated blood urea nitrogen
Posttreptococcal glomerulonephritis
Tends to occur in pediatric patients (like 3 yo) post URI
group A strep antibodies are deposited in kidney walls
URI or strep throat
dysuria, HTN
Polyarteritis nodosa
attacks medium sized muscular arteries
may be idiopathic or a type 3
typically occurs at age 40-50
Affects GI tract, heart, kidney, liver
Causes fever, pain, neuropathy, weight loss, asthma
typically results in elevated WBC, protein or blood in urine
Check biopsy of necroses area or angiogram
long term steroid therapy
Artus Phenomenon can occur with booster tetanus shots
local type III
local vasculitis of dermal blood vessels
Type IV Cell Mediated or Delayed Hypersensitivity
T cell mediated typically occur 24-92 hours post exposure
Characterized by caseous necrosis (granulomas) surrounded by giant cells, lymphocytes, and epithelioid macrophages
Disease Examples:
Infections with TB, Leprosy, or histoplasma capsulatum (fungi)
Reactions to tumors
Sarcoidosis
characterized by ground glass in X-rays
More common in African Americans
unknown etiology
may have spontaneous resolution or patients may develop chronic granulomas
some symptoms: erythema nodosum, may look like a lung infection, weight loss, malaise, fatigue, swollen lymph nodes, dry cough, fever, arthritis, cranial nerve palsy
Contact dermatitis
Remove stimulus usual resolves
me in the cadaver lab
Discuss organ transplantation and blood transfusion
Types of Transplant
Autograft
self to self transplant
no chance of rejection (its your own self)
Some people will donate their own blood to be used in pregnancy or surgery
Isograft
GENETICALLY identical twins
No rejection → complete MHC/HLA match
Homograft (allograph)
Homie to homie transplant
Must test for histocompatibility using HLA antigens
you want these as close as possible, usually siblings
Rejection chance is minimized as much as we can but its still a risk
Xenograft
Species to species transplant
like a pig heart valve
minimize risk to try to match MHCs
Transplant rejections
typically allografts
Providers need to balance immunosuppressants (cyclosporine, prograft) and other meds to avoid illness
We’ve got all types of transplants: kidney, skin, liver, heart, lung, pancreas, bone marrow
Hyperacute
usually happens during the transplant surgery
Due to preformed antibodies
Causes a clot (thrombosis) that cuts off blood flow to the organ (hypo-perfusion)
Acute reaction
typically 1-2 weeks after transplant
severe inflammation in that region, hypo-perfusion
Chronic transplant rejection
typically months to years after transplant
blood vessel damage, hypo-perfusion, die
Graft Vs. Host reaction
mediated by transplanted T lymphocytes (Donor rejects hosts)
Most often a complication of bone marrow transplantation → typically attacks multiple organs
Skin - exfoliative dermatitis
Intestine - malabsorption and diarrhea
Liver - jaundice
Blood Transfusion
The most important thing to match it the ABO type
We have natural antibodies against the opposite blood type
this prevents transfusion between groups
The second thing we need to match if the Rh + or -
Antibodies form only after sensitization
Rh Factor Incompatibility
Rh negative mom pregnant Rh positive fetus
Usually no antibodies until after the birth because we’re not sensitized
On the second pregnancy we see erythroblastosis fetalis which causes hemolysis of the fetal RBCs and kills the fetus
Anti Rh immunoglobulin (Rhogam) has be given every pregnancy to prevent sensitization
Cross matching is important before the transfusion
If we don’t cross match and guess wrong, you get intervascular hemolysis
If someone is dying and we don’t know the blood type grab O neg (AKA code blood)
AB is universal recipient, O is universal donor
Describe the major autoimmune diseases
Abnormal reactions to self antigens
When diagnosing these look for:
autoantibodies in blood
direct or indirect evidence that immune mechanism may be the culprit
Like if we try a course of steroids and it works really well I have bad news champ
Genetics play a role
Familial, linked to HLA haplotypes, sex differences
increased incidence in some families
HLA 27 is linked to anklysosis spondylysis
More common in females
Can be systemic
SLE, Rheumatic fever, RA, systemic scleosis, polyarteritis nodosa
SLE where the body makes antibodies (AKA antinuclear antibodies) against ones nuclear components
Presents with inflammatory diseases such as flomerulonephritis, dermatitis, arthritis, among others
Clinical features of Lupus
butterfly rash
arthritis
kidneys are usually involved
anemia
enlargement of lymph nodes, spleen
Usually treated with NSAIDS but not chronic steroids
Or Organ specific
MS (CNS)
Hashimotos, Grave’s (thyroid)
Autoimmune hemolytic anemia (blood)
Pemphigus vulgaris (skin)
Myasthenia Gravis (muscle)
Discuss inherited and acquired immunodeficiency diseases
Primary Immunodeficiency Diseases (Congenital)
Maybe she’s born with it
Severe Combined immunodeficiency (Bubble boy with John Travolta)
Defect of lymphoid stem cells so no Pre-Bs or Pre-Ts
Increase risk of death from opportunistic infections
Usually in Pediatric patients
Isolated deficiency of IgA
Most common (1 in 700)
patients are often asymptomatic
DiGeorge Syndrome
T-cell deficiency from Thymic dysplasia
Caused by a block in the formation of the thymus
No Ts so patients get recurrent viral and fungal
Usually presents with tetani in the first days of life
Treated with thymus or bone marrow transplant
Secondary Immunodeficiency Diseases (Acquired)
AIDs (acquired immunodeficiency syndrome)
Caused by HIV (human immunodeficiency virus) an RNA Virus
Reverse transcriptase is the enzyme the virus uses to get all up in the Cell DNA
Infects helper Ts cells so you don’t get activation of other immune cells
Macrophages and related phagocytic cells can also become infected
Treated with AZT for CD4 counts of less than 500
Patients typically presents weird infections or really bad cases
In the Lungs
Pneumocytis carinii, aspergillus fumigatus, candida albicans pneumonia
Diffuse interstitial pneumonia
In the GI tract
Candida albicans, herpes, CMV, stomatitis, esophagitis, MAI, Cyptosporidium enteritis, fungal/bacterial proctitis
CNS
toxoplasma gondii encephalitis, cyrptococcus neoformans meningititis
Chapter 4: Neoplasms
Define terms used in the field of oncology
Neoplasia - new growth, uncontrolled abnormal growth
Tumor - swelling of tissue, not specific to inflammatory event
neoplasm is the same thing
Cancer - (malignant neoplasm) uncontrolled division in a specific area
book says its just neoplasm plural
Oncology
Clinical oncology - like in the office with the patient
primarily from a diagnostic and therapeutic point of view
Experimental oncology - “tip of the spear” experimental sciences
in the lab
Cancer epidemiology - studying incidence, precedence, survival rates, treatment modalities
deal with neoplasia in human population and study the environmental causes tumor
Describe how tumors are classified
Clinical classification
Subjective/objective evaluations done in the clinic with yo eyeballs
Histologic classification
Biopsy with the all pink slides
Clinicopathologic classification
combo of clinical and histologic
Benign Tumors
The neutral tumors not good or bad
Growth is slow, expansive
No Metastases
Smooth surfaces
Has a capsule
No necrosis, no hemorrhage
Resembles normal tissue
Cells are typically well differentiated
Normal nuclei (size and shape; uniform)
Very few mitoses
Can still cause pain, but its usually due to pressure
Malignant Tumor
the bad guys
Tend to grow fast and be invasive
WILL metastasize
Can occur at any point
A primary tumor levels up and forms metastatic clones that are able to degrade lining and are cloaked from immune system
Metastatic clone can break off and enter the blood stream or lymph system
similar to inflammatory process: margination, diapedesis
Can invade other tissue or embolize (form a clot)
Early identification and destruction is key
Get your paps
tend to look insane, irregular surface
No capsule
You will see necrosis and hemorrhage
the cancer needs blood duh, remember all those little extra vessels in Franklin…
Tumor-induced angiogenesis: tumor develops its own blood supply
feed me more
Does no resemble the tissue of origin
Poorly differentiated cells
Pleomorphic nuclei - all over the place
Hypochromic
Some big or small
you may have a lot of them
Lots of bad of mitoses
Histologic Classification of Tumors
Mesenchymal tumor
Named formed by the cell or origin + oma (benign) or sarcoma (malignant)
fibroma and fibrosarcoma
This naming system is used for muscle, bone, and connective tissue
Epithelial Tumor
Named formed by using terms like adenoma/papilloma (benign) and carcinoma (malignant)
intestinal adenoma and adenocarcinoma
This naming system is used for mucosal tissue and skin
Tumors of blood cells and lymphocytes → leukemia, lymphoma, multiple myeloma
Multiple myeloma is a B cell cancer characterized by bony punchy lesions
Bone marrow is basically only plasma cells
IgG
Misc.
Tumors of neural cells → ganglionneuroma, neuroblastoma
Germ Cell tumor → teratoma, embryonal carcinoma, seminoma/dysgerminoma
Teratomas are derived from germ cells and contain multiple tissue that are formed from all 3 layers (ectoderm, mesoderm, endoderm) Not metastatic
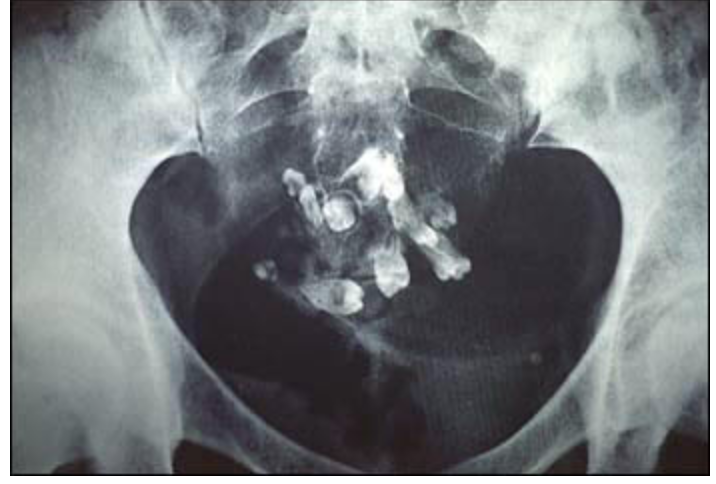
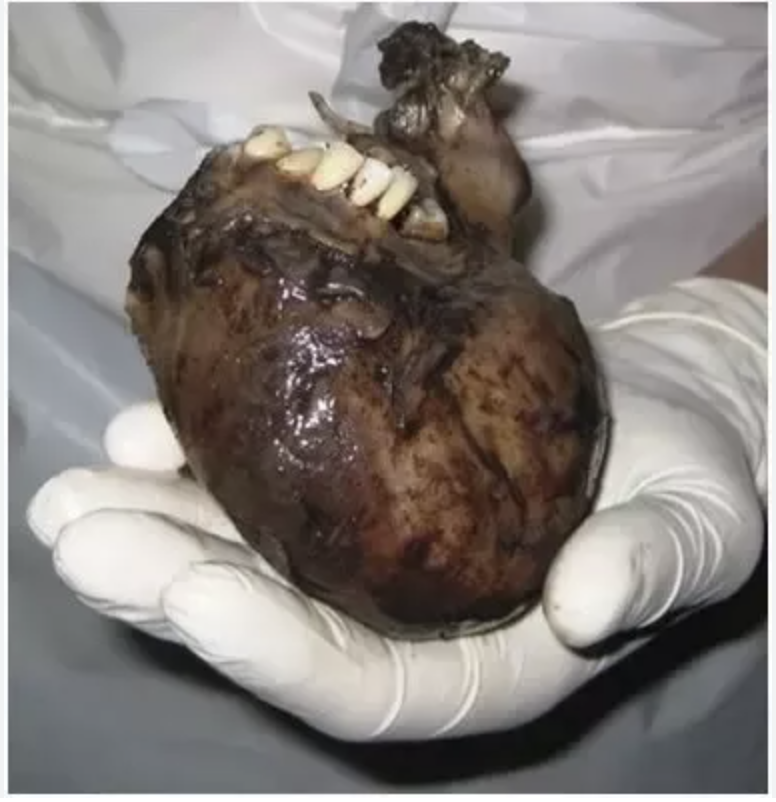 Since teratomas are from germ cells you can get weird stuff like teeth and hair and I love them
Since teratomas are from germ cells you can get weird stuff like teeth and hair and I love them
Blastoma: malignant tumors composed of embryonic cells originating from embryonic primordia
retinoblastoma - eye
neuroblastoma - adrenal medulla or immature neural cells
hepatoblastoma - liver
nephroblastoma - kidney
glioblastomas - brain
Named after Dude Bros (Eponymic tumors)
Hodkin’s - lymph nodes
Ewing’s - long bones
Kaposi’s - on the skin, usually AIDs patients
Stagin’ and Gradin’
Staging: based on clinical assessment during gross exam, surgery, x-ray, etc.
Uses the TMN system
T - size of tumor
1-4 low is good
N - lymph node metastases
1-4 low is good
M - distant metastases
0-1 low is good
Grading: based on histologic exams
Discuss the causes of cancer
Exogenous Causes
Chemical exposure from occupation or environment
Physical from like the sun, pollution tobacco, alcohol, diet
biologic from viruses
Endogenous
Oncogenes - proto-oncogenes are reproduced
could be familal
Tumor Suppressor genes - breakdown in suppression system
Wombo Combo
9/10 its usually an interaction between the 2
When combined there’s exponential increased risk
Describe the clinical manifestation of Neoplasia
Clinical features depend on type of tumor, location, grade, stage, immune status of host, sensitivity of the tumor cells to therapy
Systemic Symptoms
Cachexia (generalized weakness)
caused by wasting
Weight loss and loss of appetite (anorexia)
Neurological symptoms
Can have obstruction of airways or digestive tube
Can have have respiratory dyspnea and pneumonia
Splenomegaly
Intestinal obstruction
Abdominal masses
skin lesions
Liver enlargement
ascites (swollen stomach)
Bleeding in Urinary tract, rectal, vaginal
thrombosis
Malaise (general fatigue)
Paraneoplastic Syndrome
Paraneoplastic syndrome: substances that are secreted by cancer cells that affect other systems
small cell carcinoma of the lung→ Cushing’s Syndrome
May produce ACTH and overstimulate the adrenals
squamous cell carcinoma of the lung → Hypercalcemia
hyper PTH
renal cell carcinoma → Polycythemia
renal cells release hella EPO and we get way more blood
pancreatic carcinoma → Venous thrombosis
Release of thromboplastin
Thymoma → myasthenia gravis
tumor in the thymus blocks ACH receptors as neuromuscular junctions which impairs transmission obviously
Chapter 9: Heme and Lymph Pathology
Describe the Composition of peripheral blood*
Blood is made up of hematocrit (RBCs), Buffy coat (WBCs, platelets), and Plasma (water, proteins, nutrients, hormones, electrolytes, clotting factors)
Humans have around 5.5-6L of blood
Normal blood hematocrit in females is 37-47%, in males its 42-52%
Erythrocytes
120 day lifespan
Made in the bone marrow, recycled in the spleen
4 heme group, 4 globins
O2 and CO2 bind to the iron in the hemoglobin
To make hemoglobin you need: iron, B6, B12, and folic acid
Normal hemoglobin levels are 14 in females, 15.5 in males
A normal reticulocyte (baby RBC) is 1-5% of all RBCs
MCV (mean cell volume) is how big the cells are
low = microcytic
high = macrocytic
MCH (mean corpuscular hemoglobin) is the average hemoglobin on 1 RBC
MCHC (mean corpuscular hemoglobin) is the average hemoglobin in the total volume
Basically MCH/MCV
A low MCH or MCHC indicates hypochromic
Leukocytes*
Neutrophils are the most common at 60-70% in blood
Defense against bacteria infections
survive for like 4 days
React to chemotaxis → high motility
Phagocytes
Lymphocytes are found at 25-33%
Bs, Ts, NK, and stem cells
Last longer than neutrophils
Viral infections, mono, whooping cough
Monocytes are found 3-9%
Precursor to macrophages
Last longer that neutrophils
malaria, TB, fungal infections
Eosinophils are found at 1-3%
Allergic reactions
Parasites
autoimmune diseases
Basophils are found at less than 1%
allergic reactions
Granules contain histamine, heparin, serotonin
cancer, chicken pox, hypothyroidism
Thrombocytes (platelets)
essential clotting factor
survive like 10 days
Anemia*
Anemia may result from
Decreased creation of RBCs (hematopoesis)
Abnormal hematopoesis
think genetic
Sickle cell (most well known) poorly shaped erythrocytes cannot function properly so patient is always hypoxic
Increase loss or destruction of RBCs
hemorrhage
Over active spleen
hemolysis
infections (like malaria)
in Malaria bacteria invades RBC and causes their lysis
Decreased Production of RBCs (the basics)
Aplastic Anemia (bone marrow failure)
Pancytopenia
typically idiopathic
Myelofibrosis
Begins as a myeloproliferative disease and leads to scarring in the bone marrow
Anemia can be the result of bone marrow being replaced by cancer cells
Nutrient deficiencies (iron, b12, folate, protein)
Iron is the most common nutrient deficient
Microcytic hypochromic
Can be made worse by intestinal malnutrition syndrome
B12 and folate are essential for DNA synthesis and maturation of hemopoietic stem cells - low levels cause megoblastic anemia
No protein no cells duh
Nutrient deficient anemias are markers for starvation and malnutrition
Anemia Morphology (the basics)
Normochromic Normocytic
RBCs are pretty basic
This type of anemia is usually due to hemorrhage or anemia of chronic disease
Microcytic Hypochromic
RBCs are small and pale
Iron deficiency
Thalassemia
Macrocytic Normochromic
Normal color but BIG cells
B12 or folic acid defiency
Liver disease
If the shape of the RBCs is the issue (chronic hypoxia and reduced RBC lifespan)
elliptocytosis (oval shaped)
Sickle cell
spherocytosis (ball shaped)
Let’s talk diseases baby*
Iron Deficiency Anemia
most common form of anemia, typically associated with depletion of ferritin
Remember no iron, no Heme
Caused by the
Increased loss of iron (bleeding)
periods, ulcers, polyps, NSAIDS, hookwormds, injury
Inadequate intake/absorption (bad diet or GI issues)
increase use of antacids
intestinal disorders (Crohns, Celiac)
Increased iron requirements (preggo)
Pathogenesis
Iron is absorbed in the intestines and bound to transferritin (the uber for iron) or ferritin (storage form)
Ferritin aggregates = hemosiderin
test with prussian bliue
Iron is lost usually through cell lost
recycled in spleen
Shed through desquamation
menstrual bleeding
Pathology
Microcytic Hypochromic normal hematopoeisis
Clinical Quirks
more common in females
if you see it in males think occult bleeding
Symptoms are palor, weakness, and sometimes in kids you’ll see pica (like they want to ear dirt)
Labs: CBC, iron, B12, folate, TIBC, transferritin, ferritin
Treatment
iron supplements
stop the bleeding if necessary
Megaloblastic Anemia (B12 or folic)
Reminder: gastric bypass patients have trouble absorbing B12 and since B12 is only found in animal products vegans must take supplements
Most severe form is pernicious anemia which is a lack of intrinsic factor (IF)
Pernicious Anemia: atrophic gastritis and lack of intrinsic factor, antibodies may prevent the binding of IF to B12
May also be caused by celiac, Crohn’s, parasites, etc.
Crohn’s affects the part of the intestine where B12 is absorbed
Pathology
CBC shows decreased RBCs but they big
In pernicious anemia, bone marrow is hypercellular and there’s lots of megaloblast
Clinical quirks:
Same as all the other anemias
Maybe some spinal cord involvement if it gets really bad
loss of vibration, proprioception, deep tendon reflexes
May persist even after treatments
Treatment
B12 injections or sublinguals
Folate supplements
Aplastic Anemia (Bone marrow ain’t working)
Typically idiopathic in nature or can be due to chemo/radiation/infection
Pathology
Bone marrow is scarred - consist of fibroblast, fat cells, and scattered lymphocytes
Clinical Quirks
recurrent infections due to pancytopenia and bleeding
Basic anemia symptoms
Treatment
get a new bone marrow
about 60% improve!
Hemolytic Anemias
RBCs can be destroyed because of structural abnormalities or because of antibodies, infectious agent, mechanical factors
Common features of hemolytic anemias: reduced RBC life span, increase of erythropoetin (trying to compensate), increased reticulocytes, hyperbilirubinemia (more dead RBCS = more bilirubin = jaundice)
Sickle Cell
A result of a point mutation of the HbA results in the HbS (gotta be homozygous to have the disease)
Those with above 80% HbS show all the typical symptoms
40-80% HbS means symptoms are mild to moderate
Under 40% your asymptomatic congratulations
Most common in African populations (30%)
HbS undergoes polymerization (they stick together) at low oxygen tension, so we get “sickling”
this can occlude small blood vessel and cause ischemia
Gotta avoid high altitudes, strenuous exercise,
Sickle cells are destroy in the spleen → increase bilirubin
Symptoms start at age 1-2
Sickling Crisis are usually induce/aggravated by fever, respiratory diseases, anoxia
Thalassemia
Caused by a genetic defect in synthesis of HbA so less globin is made
T-beta: less beta chain is made
worse and more common
T-alpha: less alpha chain is made
T _____ minor: one of four chains effected, heterzygotes
T _____ major: severe usually lethal
Children usually die due to lack of hemoglobin unless transfused
Usually of mediterranean descent
Clinical quirks
Microcytic Hypochromic
T-major results in hepatosplenomegaly, iron overload
Slow growth
There’s no cure
Hereditary Spherocytosis
Genetic defect of structural proteins of RBCs (looks like a ball)
Destabilization of membrane and thus lysis in spleen
Autosomal dominant 1 in 5000 white people
The balls can not adapt to microcirculation, if there’s a vasoconstriction the ball cannot go through
Clinical Quirks
typical anemia
splenomegaly and jaundice
Treatment
Get rid of the spleen (does NOT help the RBCs in microcirculation)
Immune Hemolytic Anemia
IgG binds to a RBC autoantigen, activating complement which lyses RBCs (hypersensivity type II)
idiopathic or due to drugs/environment
Polycythemia
Typically due to clonal proliferation of hematopoietic stem cells, myleoproliferative disorders, NEOPLASTIC.
In secondary polycythemia you see increase erythropoietin
Basic clinical features: easy clotting, HTN, appeared flushed, neuro symptoms, erythroid hyperplasia in the bone marrow, Vera (bone marrow cells look off)
Typically treated by
Phlebotomy, blood letting
In the case of Vera, use chemo drugs
Leukopenia
Most important are neutropenia (agranulocytosis) and lymphopenia
Neutropenia = low neutrophils
Lymphopenia = low lymphocytes
Selective lymphopenia is like a specific type (AIDS, CD4s)
Maybe caused by chemo, environmental and industrial chemicals, radiation and some chronic diseases damage the bone marrow
can be a part of aplastic anemia\
Clinical Quirks
Neutropenia: recurrent bacterial infections
Lymphopenia: frequent bacterial, viral, fungal, and or parasitic infections
long term leukopenia and aplastic anemia are often fatal
Treatment
stop exposure
give poietins
Leukocytosis (white count)
Etiology and pathogenesis
Small increase due to fighting infections so usually benign
Neutrophils are high: bacterial infection
Eosinophils are high: parasite
Lymphocytosis: usually viral, chronic infections (TB), autoimmune
Sometimes its just inflammation
Swollen lymph nodes in URI, EBV, and early AIDS
If the lymphadenopathy is persistent get a biopsy
WBC and Plasma Malignancies*
Etiology and pathogenesis
idiopathic or can be caused by a virus/activation of an oncogene
EBV - flu like symptoms, mono or burkitt’s lymphoma (children/sub-saharan Africans)
Human T Cell leukemia/lymphoma virus (HTLV-1)
Can be injected and causes lymphoma so its oncogenic
T-lymphotropic virus - same fam as HIV
Burkitt’s lymphoma can also be caused by translocation of chromosomal fragments 8 and 14
Philly Chromosome (short 22) can cause chronic myelogenous leukemias
Clinical Quirks
Bone marrow infiltration
increased number of immature blood cells
neoplastic stem cells show genetic changes
Anemia
recurrent infections
usually cause of death
uncontrolled bleeding
Note: children leukemias are often acute, adults are chronic
Acute Lymphocytic Leukemia
Myeloproliferative disease that peaks in patients at the age of 5
incidence however rises in old people
Most common form of leukemia in kids
Rapid progression
66% cure rate
without treatment though its lethal in 3-6 months
Chemo
Clinical Quirks
bone pain
recurrent infections
weakness
bleeding into the skin/major organs
lymphadenopathy
splenomegaly
Acute Myeloid Leukemia
Most common leukemia in adults (40% of all leukemias though)
mostly elderly patients
at least 20% malignant myeloblast in bone marrow
Lethal in 6 months without treatments
with treatment (chemo) 5 year survival is 15-30%
Characterized by AUER RODS on smear
Chronic Myelongenous Leukemia (CML)
15% of all leukemias
rare before adolescence and incidence increases with age
disease of pluripotent stem cells
Most patients die in 3 years
Better Prognosis if the patient has philly chromosomes
Treatment: chemo, radiation, bone marrow transplant (lead to a 70% chance of 3 year survival)
Gleevec is promising and causes remission in 90%
Clinical Quirks
Slow onset
mild anemia
hypermetabolism
fatigue
recurrent infections
splenomegaly and clotting are common
Phase of CML
Chronic 2-3 years
marked leukocytosis of eosinophils and basophils
<10 blast in bone marrow on biopsy
increase platelet and megakaryocytes (platelet precursors)
Accelerated (50% of patients)
>10% blast in bone marrow
typically ends in blast crisis
>20% basophils in blood
unresponsive to treatment
Sudden onset (other half)
cannot be treated
Chronic Lymphocyte Leukemia
25% of all leukemias found in old people
Slow progression (7-9 years)
Can transform which lessens our prognosis
Clinical Quirks
SMUDGE CELLS in smear
indistinguishable from normal lymphs
reduced infection resistance
Treatment
unresponsive to chemo
Lymphomas *
3% of all malignant diseases
no such thing as a benign lymphoma
*Most lymphomas have a B cell Phenotype*
More common in adults but happen at all ages
Malignant cells often infiltrate primary lymphoid tissue
Diagnose with lymph node biopsy, immunohistology, Flow cytometry, genetic analysis
Non-Hodgkins Lymphomas (NHLs)
Most often involve lymph nodes, bone marrow, spleen, thymus but can also be extranodal
Spill into blood → lymphoblastic/lymphocytic leukemia
Clinical quirks
Lymph node enlargement (lone wolf or pack life)
Fatigue, malaise, weight loss, hypermetabolism, anemia, leukopenia, recurrent infections, autoimmune phenomenon
Extranodal tumor spread → brain with multiple neuro symptoms
Follicular
Most common in US in elderly
slow growing
usually mild symptoms
chemo is not effective
at terminal stage body is overwhelmed by tumor burden
Diffuse large B cells (DLBL)
Most aggressive NHL with several forms
tumor cells spread
complete remission in 75% with chemo
Burkitt’s
Highly malignant
cells prone to apoptosis
chemo works good and can almost completely cure
Common in sub-Saharan Africa due to EBV
Tumor of mandible/face
Outside endemic
rare but affects kids and young adults
abdominal mass
Hodkin’s Lymphoma (HL)*
Rare monoclonal lymphoid neoplasm with 4 feature
presents in young adults
Peaks at 25-55
cervical lymph nodes
involves scattered large mononuclear Hodkin and multinucleated Reed-Sternberg Cells* on a background of non-neoplastic inflammatory cells
Characteristic neoplastic cells are often surrounded by T cells
5 types
Nodular Sclerosis
Mixed cellularity
lymphocyte predominance
lymphocyte depletion
lymphocyte rich
Prognosis depends on spread, staging is important
Stage 1: 1 region
Stage 2: 2 or more lymph node regions same side of diaphragm
Stage 3: involvement on both sides of diaphragm
Stage 4: extranodal tissue involvement
Multiple Myeloma*
Malignant disease of PLASMA cells
a single cell undergoes a malignant conversion
clonal expansion, so its monoclonal
Disease of old age (>45)
Normochromic anemia
Mild leukopenia
Thrombocytopenia
Bone fractures common
Quirks:
Bence jones protein in urine
*Punched out bony lesions in calvaria, vertebrae, long bones on xray*
Hypercalcemia
calcium is released from bones and deposited in kidneys
Renal failure → typically what kills the patient
Diagnosis
based on xrays, serum electrophoresis, bone marrow biopsy
Prognosis
grim, chemo is ineffective
Vascular Disorders
Mechanical trauma causing small bruises, wounds, hematomas
Vessel wall weakness
depends on tissue strength
as we age it gets worse (Senile purpura)
Scurvy marked by multiple hemorrhages (remember the PINK song from Spongebob)
intracellular matrix of vessels require vitamin C
Immune mechanism can damage vessels
Platelet Disorders*
Quantitative: decrease number
Qualitative: abnormal structure or function
Congenital or acquired
Aspirin prevents platelet aggregation and factor 3 release
Thrombocytopenia is < 100,000 platelets
Prolonged post-op bleeding or spontaneous bleeding
Decreased production from
aplastic anemia
leukemia
drugs that damage megakaryocytes
infection (rubella)
Treatment
transfusion of blood or platelets
but you have to fix the problem
Increased Destruction
Note: A transfusion reaction can cause hemolytic anemia
autoimmune, drug induced, ITP, maternal paternal platelet antigen mismatch
Idiopathic thrombocytopenia purpura: idiopathic, maybe autoimmune
DIC (disseminated intravascular coagulation)
Consumptive coagulopathy
We clot a lot and use up all of our clotting factors so if a bleed occurs theres’s none left
The clots can often cause ischemia or shock
Labs:
low platelets
Elevated D dimer
Decreased fibrinogen
prolongation of PT/PTT
Hemophilia
Congenital is common but can also be aquired
Hemophilia A = no Factor VIII
mild, moderate, severe
Hemophilia B = no factor IX
X linked disease so women can be carriers but men only need one copy to be affected
New mutations are 20% of cases
prolonged aPTT
there’s specific test to distinguish A or B
Clotting Factor Deficiencies
chronic liver disease affects most clotting factors (they’re made in the liver)
Vitamin K is needed for Factors II, VII, IX, X
Vitamin K is produced by gut bacteria and transported to blood stream with fat cells
Deficiencies can be caused by antibiotics, the inability to absorb, pancreatic disorders, warfarin