Chemistry Midterm 1
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms

potential energy for two charges; like on top and opposite on bottom. like: less PE as you get further but a great amount of PE as you get close bc pushed so closely they can repel in opposite directions; opposite: greater PE as get further from each other
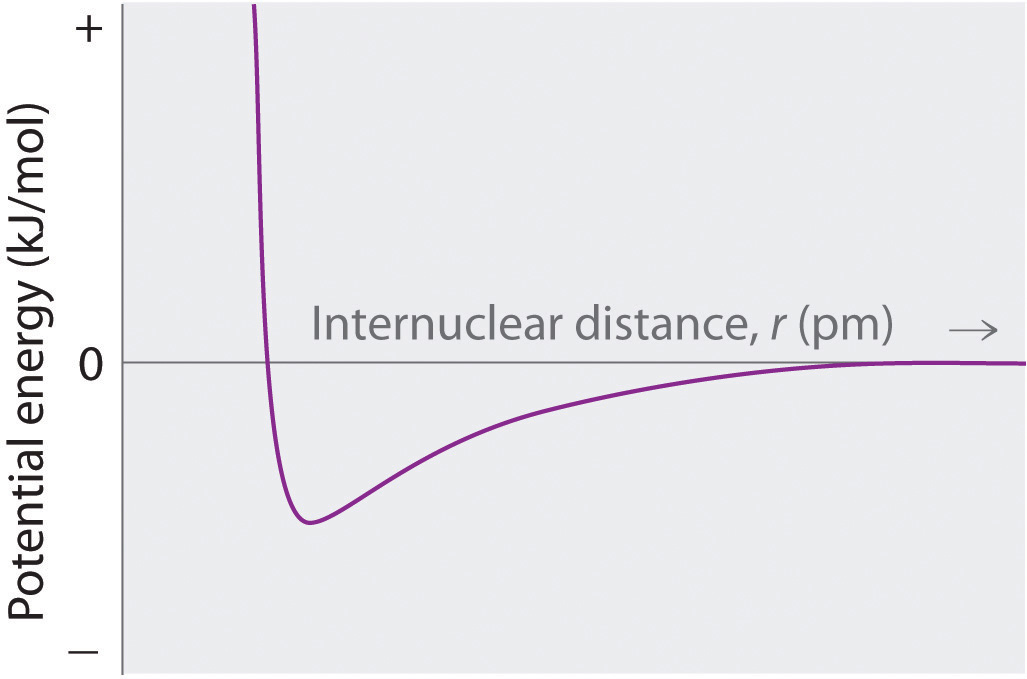
PE dissociation curve for covalent bond (in the neutral state); breaking up into atoms; distance between ditch and x axis is energy required to break bond; distance from ditch to y axis is the equilibrium bond length; straight ish curved line under x axis to the right is where attraction and bonding happens
ionic bond
normally metal and nonmetal; transferring electrons from one atom to the other, creates a cation and anion
covalent bond
sharing of electrons; H2; normally nonmetals

PE dissociation curve for ionic bond; separating bond into charged ions; hump comes above x axis because it costs energy to transfer electrons; distance between ditch and x axis is energy required to break bond; distance from ditch to y axis is the equilibrium bond length; writing eq: AB→A+ + B- e-+ A+→ A B- →B+e-; cross out; AB→A+B; add delta Es together; top is coulombs law; mid is -IE (bc flipped); bottom is EA
what does atomic number tell us
number of protons; to find e- , subract/add based on charge, to find n, subtract by atomic mass
cathode ray experiment
way to prove that electrons have a (-) charge and are basically massless; cathode ray (made up of e-) is sent through electric field to accelerate e from - to + charge, then beam goes through electric field (charged plates; field points from positive to -) and is deflected by - plate, so a tangent line forms; the coils from the magnetic field is then used to tune the beam back to normal, which helps us know the angle to calculate mass:charge ratio; found all e have same mass:charge ratio, and the mass of atoms is mostly made up of + charge
plum pudding model
theorized that atom was made up of a medium of positively charged atoms and there were (-) scattered around; tested using scattering experiment and disproved
scattering experiment
+2 particles sent through gold sheet; some particles were slightly deflected and some when straight through; however, some particles deflected right back, meaning that the (-) charges must be tightly packedl there must be so many e in one space to fully deflect bc like charges will repel especially when there are a lot in one place
oil drop experiment
used to estimate charge on e; oil droplets sprayed in low humidity vacuum, and friction causes droplets to gain charge bc so many other droplets; droplets enter chamber through tiny hole where 2 charged plates exert constant net electric field; the attraction between (-) charged droplet and + plate counteracts gravity; and tune strength of electric field so that the droplets become static and a net force exerted is zero, meaning the mg=qE ; all droplets come as a common factor, so can divide to find an average.
formal charge
#of valence e - # of e in lone pairs - 1/2(# e in bonding pairs); the best structure is the one with the formal charge closest to zero
mass spectrometry
how neutrons were discovered; cathode ray tube except shoots (+) ions; divides ions based on their mass:charge ratio (isotopes); ions with lighter masses are faster and become more deflected; neutrons were proved to exist because when the same charge is exerted on ions with different masses but the same charge, there must be neutral ions in the middle (adding to mass but making no change on the charge); tells us relative isotope abundance
ionization energy
energy required for an e to be blasted off of nucleus orbit: X- → X + e-; we can find ionization energy graphically by plotting potential energy vs radius; as you go across (relatively) more ionization energy is needed bc shells filling up; IE = deltaE = Ef-Ei ;
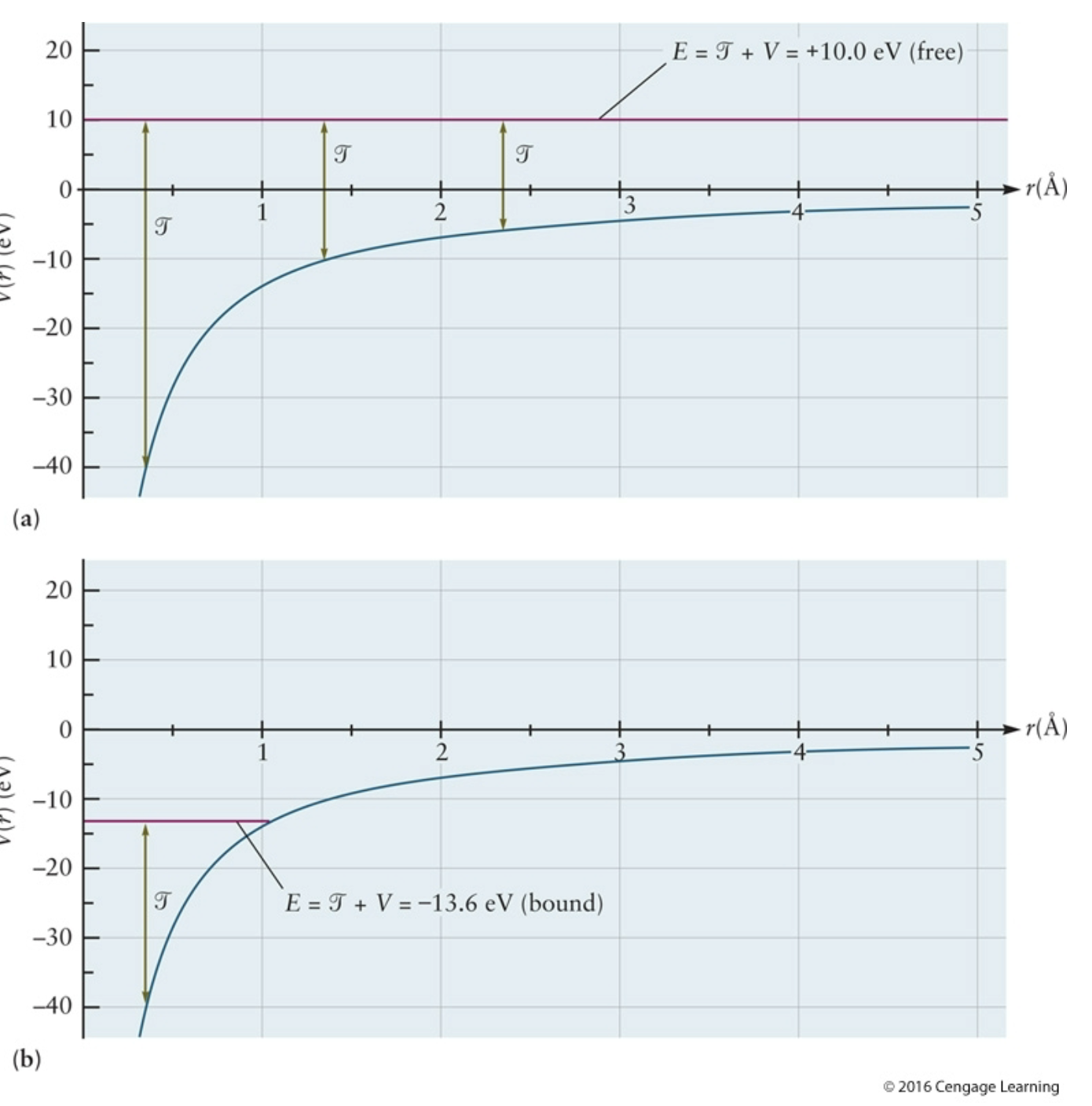
top
potential energy curve for electron in H unbound; shows a free electron- ionized; the total energy line is above zero, positive, meaning it is not bound to the atom; PE because not bound
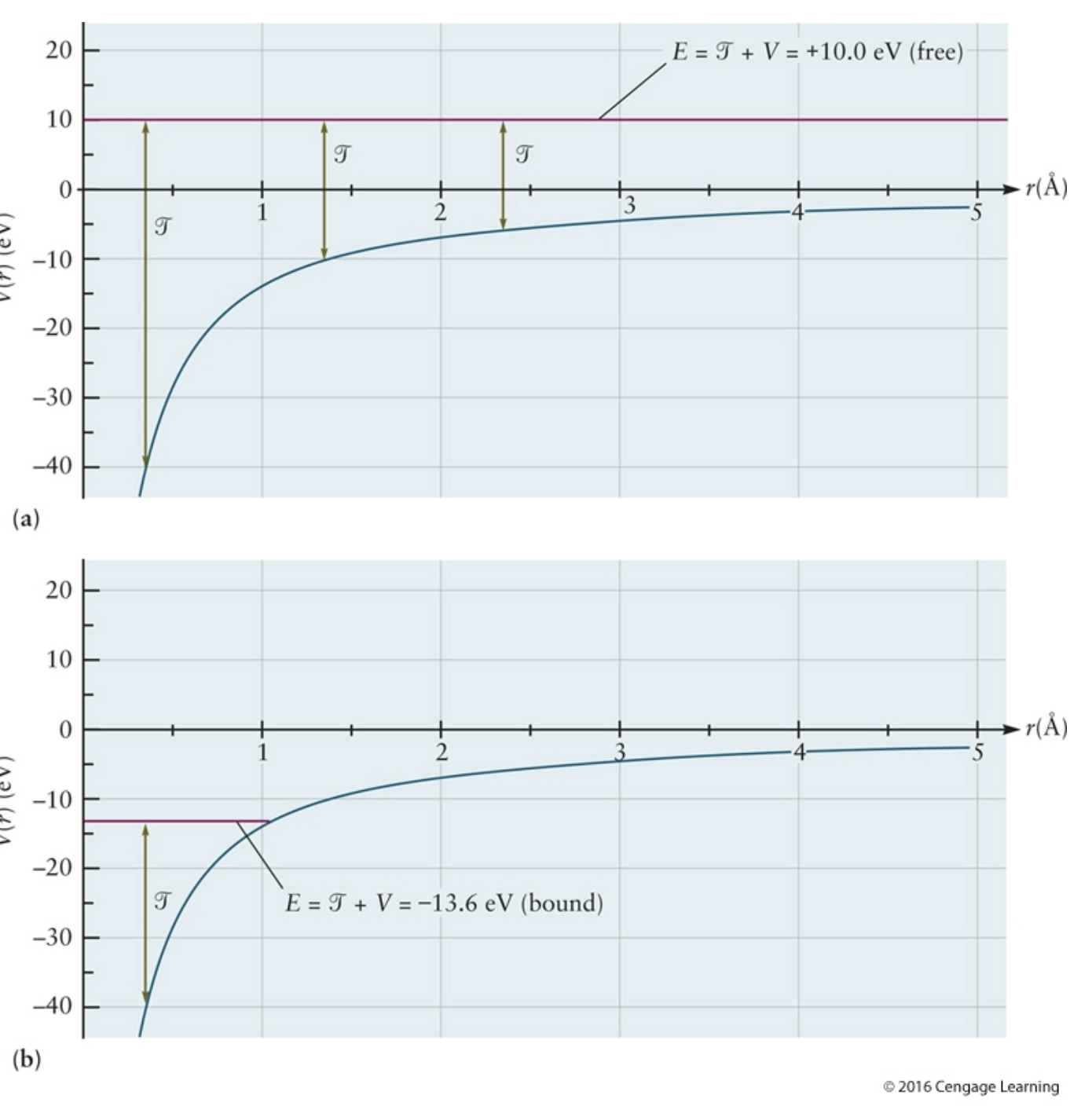
bottom
potential energy curve for electron in H bound; shows a bound electron; total energy is below zero, meaning the electron is bound to nucleus
electron shielding
every time new row starts on p table, new shell starts, so less ionization energy needed; outer/valence electrons are more weakly bound and thus less likely to participate in bonding
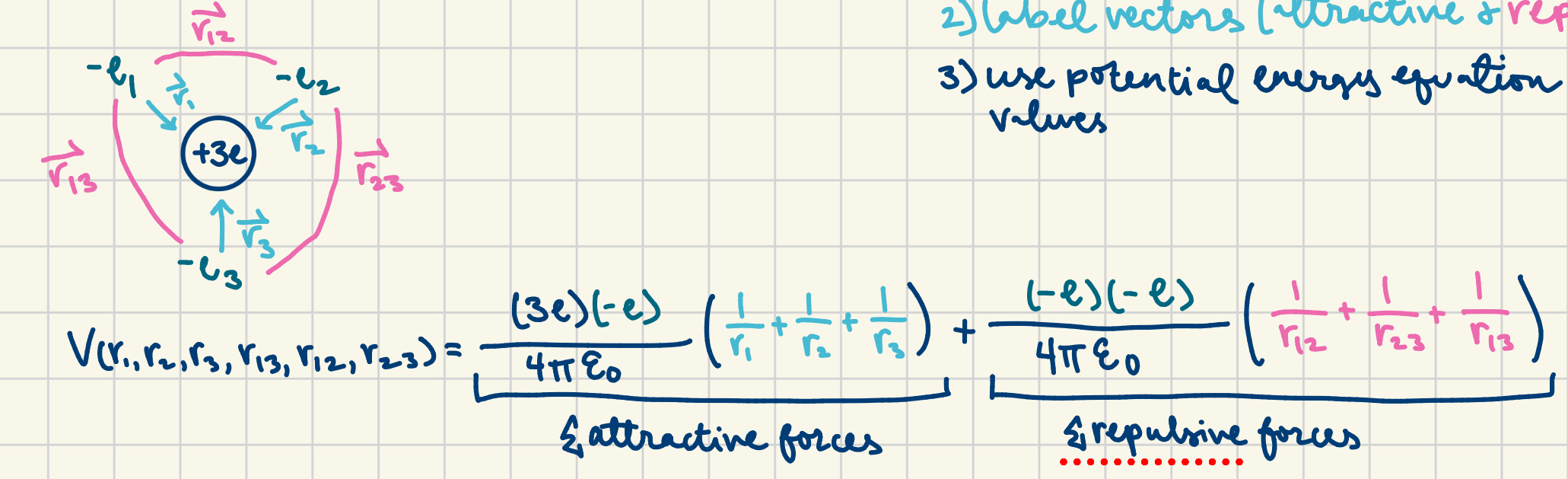
finding potential energy to understand the stability of an atom
draw configuration, label vectors (attractive and repulsive), use potential energy equation and insert values; make sure to add the attractive forces (as a one section) and the repulsive forces as the other; the more negative the potential energy, the more stable
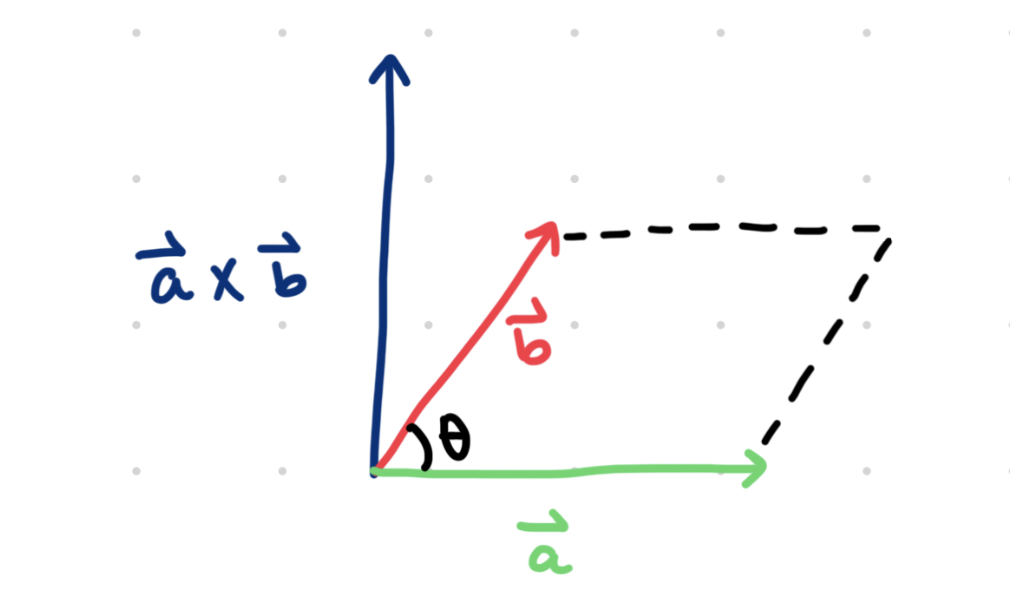
right hand rule
The cross product of two vectors results in a third vector (c) that is perpendicular, or orthogonal, to the first two vectors (a and b). Because vector c is orthogonal to pair vectors a and b, the plane consisting of all three vectors becomes three-dimensional, as represented below:
As stated earlier, vector a cross vector b must be perpendicular to vectors a and b. This resultant, however, can point upwards in the positive direction (as shown in the drawing above), or point downwards in the negative direction. A method called the right-hand rule provides a practical resolution to this. Holding up your right hand, point your pointer finger in the direction of a and your middle finger in the direction of b, then your thumb will point in the direction of vector a cross vector b.
electron affinity
the energy it takes to add an e to a configuration; X+e- → X-; depends on the elements electron configuration; Li: 1s2 2s1; not fully filled shell so easily add e so becomes more stable
duet rule
H and He obey; only 2 valence e
octet rule
F, N, O, C; obey octet rule
are double bonds 2x stronger than single bonds
no, not exactly 2x stronger but still stronger
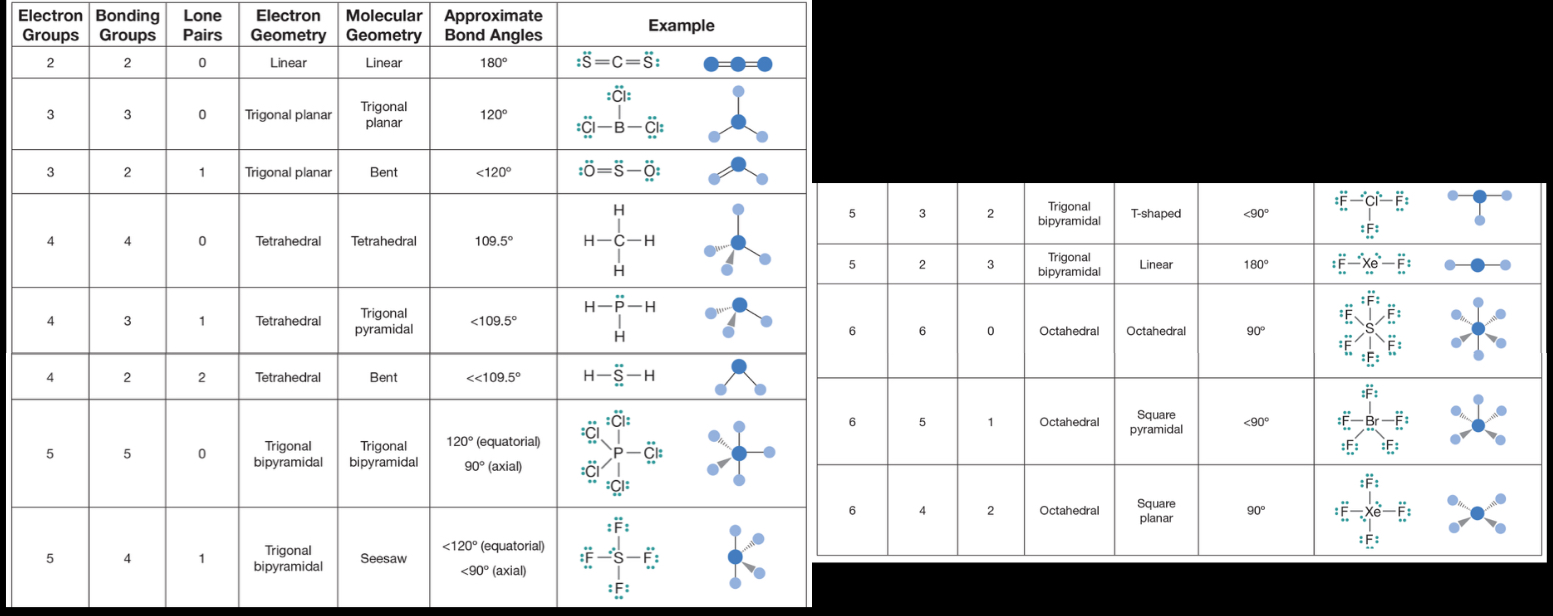
3D structures of molecules
normally polar if LP on central atom or different types of atoms around central atom
dipole moments
vector sum of all bonds; resultant vector; mu tot = ( mu1 + mu2)(cos theta)- angle made with reference between two vectors; cancellation between vectors means non polar; mu = q(d)
bond order/bond strength
looking at covalent vs ionic (both with same bond order), the bond dissociation energy is about the same, so their bond strengths are about the same; however, the higher the bond order, the greater bond dissociation energy required- higher order bonds between sim sized atoms tend to be shorter than lower bond orders
particle in a box- classical 1D
dont need vectors, one component; if you know initial location, you can know where it is at all times; when looking at time dependent (not knowing where it is initially) you have to find probability of a particle in a certain area; the faster a particle moves, the less likely to find particle at a certain place and time, however there is uniform probability density; potential energy = 0 but turns to infinite when hits wall and then back to 0; uniform probability density:

particle in a box- classical 2D
0 potential energy; uniform probability density (1/L)— straight line on p(x) graph
according to CM, why should electron spiral into nucleus
as a charged particle moving in a circle, it undergoes acceleration, and according to Maxwell's equations, any accelerating charged particle must continuously emit electromagnetic radiation This radiation carries away energy, causing the electron's orbit to shrink, leading to a rapid collapse into the nucleus
photoelectric effect
ejections of electrons from a material when light of a sufficient energy (frequency) strikes its surface; energy comes in packets called photons; E(photon) = hv planks times wavelength; if hv is high enough, e will eject; increasing the intensity does not knock e off, frequency must be raised (which is why particles must be wavelike ; to find KE=Ephoton-work function
blackbody radiation
light rays heat up object; energy inputed at high frequency and low wavelength then the body re-emits radiation based on its temperature; energy is emitted in photons; as a black body heats up, it emits radiation at different wavelengths;
hydrogen atom - classical
electron orbiting H proton; since moving in circle, the e is continuously accelerating, and a classical accelerating ion should continously emit electromagnetic radiation, causing it to spiral into the nucleus; use angular frequency
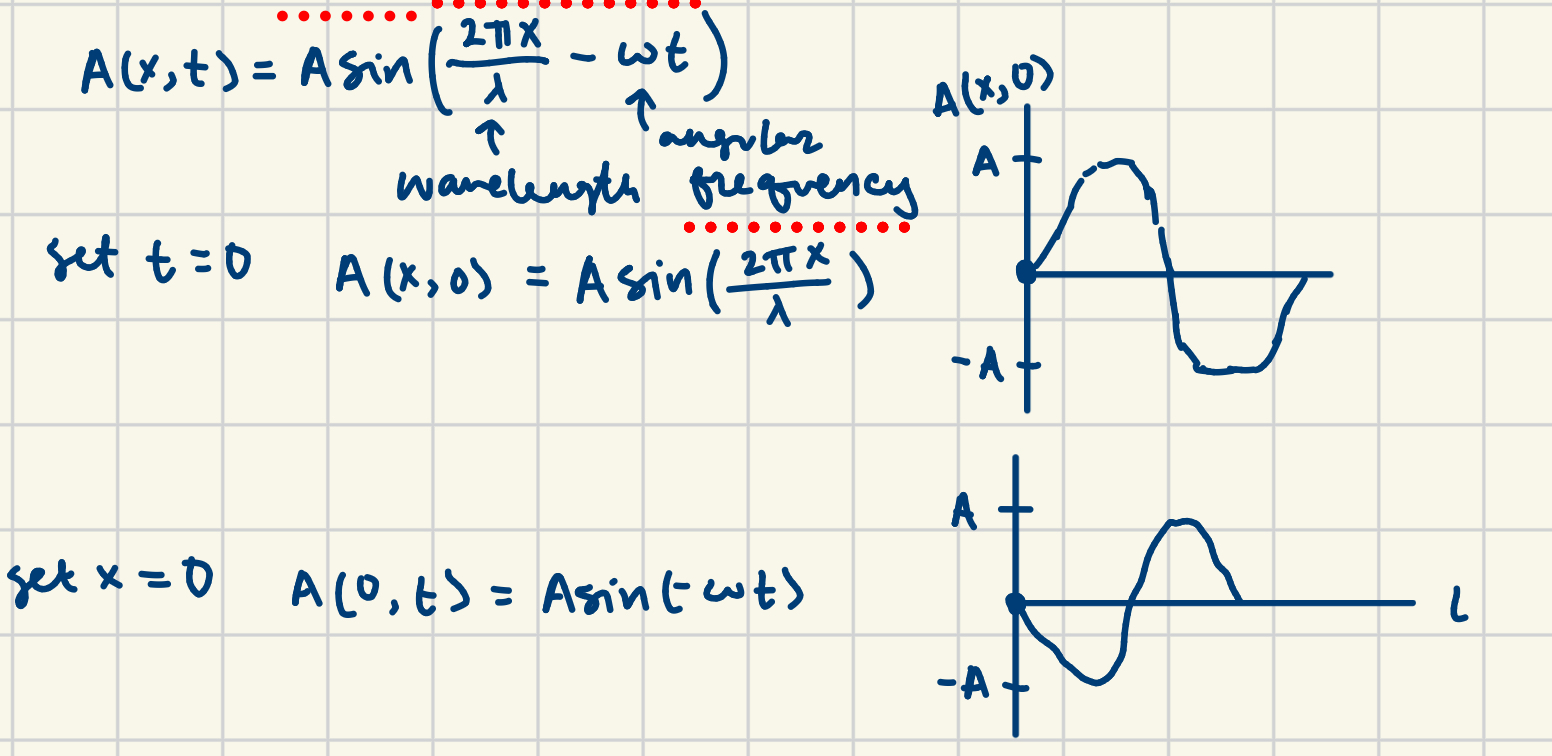
light- classical
light is a transverse wave will perpendicular electric and magnetic fields; waves interfere (add at the electric and magnetic); wave equation: speed of light = frequency(wavelength) ; to find light interference patterns, add each waves electric field then square ; accelerating charge radiates light, and frequency of emitted light = frequency of orbit; finding amplitude:
harmonic oscillator- classical
total energy always conserved; force is linear; frequency depends on stiffness of coil and mass of particle; speed is not constant; p(x) only depends on amplitude; potential energy looks like parabola and p(x) looks like boat ish
De broglie wavelenght
Used to find wavelength of a moving particle; wavelength= h/p= h/mv