Atomic structure
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
An atom is…
The smallest unit of matter that makes up an element
A molecule is…
2 or more atoms chemically bonded together
What are the 3 sub-atomic particles?
Protons
Neutrons
Electrons
What is the relative mass, relative charge & position of protons?
Relative mass: 1
Relative charge: +1
Position: In the nucleus
What is the relative mass, relative charge & position of neutrons?
Relative mass: 1
Relative charge: 0
Position: In the nucleus
What is the relative mass, relative charge & position of electrons?
Relative mass: 1/1836
Relative charge: -1
Position: In electron shells orbiting the nucleus
Describe the structure of an atom
Atoms have a nucleus which contains protons & neutrons
The nucleus is orbited by electrons shells which contain electrons
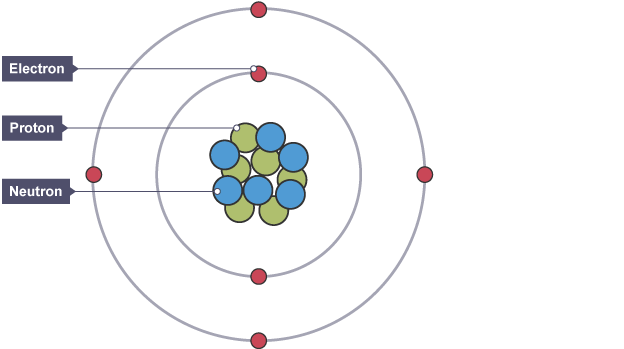
How many electrons can go into each electron shell?
1st electron shell → 2 electrons
2nd electron shell → 8 electrons
3rd electron shell → 8 electrons
The mass number is…
The total number of protons & neutrons an atom has
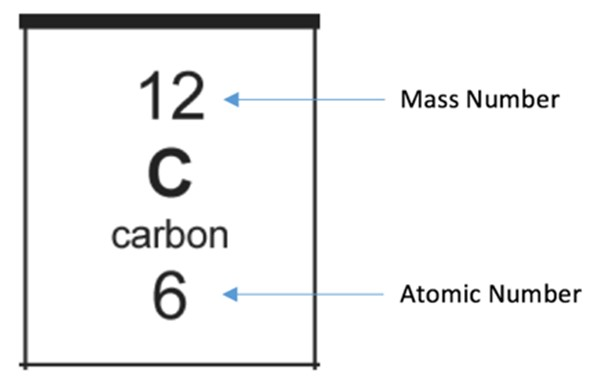
The atomic number is
The number of protons at atom has
(number of protons = number of electrons because atoms are neutral)
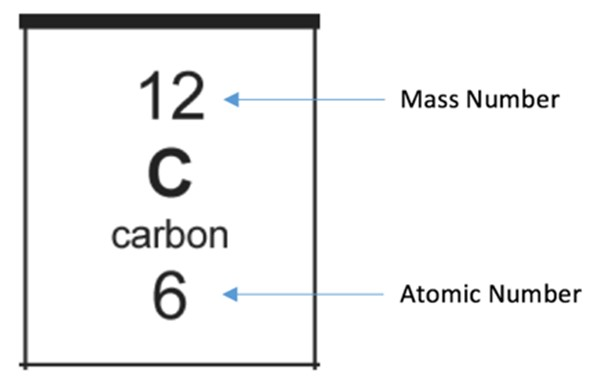
An isotope is…
Atoms of the same element that have the same number of protons but a different number of neutrons
This means that the atomic number is the same but the mass number is different
How is the relative atomic (Ar) mass calculated?
(isotope A mass × isotope A abundance %) + (isotope B mass × isotope abundance B %) ÷ (total number of atoms)