using resources topic 10
1/33
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
34 Terms
whats ceramics, what can some be made from & whats that, what happens when its fired at high temps, another example, whats mode made form & how, whats borosilicate glass
ceramics → non-metal solids w high melting points that arent mare from carbon-based compounds
some ceramics can be made from clay → soft material when its dug out of ground so can br moulded into diff shapes
when its fired at high temps it hardens to form clay ceramic
another example of ceramic is glass → generally transparent, can be moulded when hot & can be brittle when thin
most glass made is soda-lime glass, which is made by heating mixture of limestone, sand & sodium carbonate (soda) until it melts. When mixture cools it comes out as glass
borosilicate glass has higher melting points than soda-like glass. Made in same way, using mixture of sand & boron trioxide
whats composite & 4 examples
composites → made of 1 material embedded in another. Fibres/ fragments of material (reinforcements) r surrounded by matrix acting as binder. Properties of composite depend on properties of materials its made from
Fibreglass consists of fibres of glass embedded in matrix made of polymer (plastic). Has low density (like plastic) but v strong (like glass). used for things like skis, boats, surfboards
Carbon fibre composites also have polymer mix. Reinforcement is either made from long chains of carbon atoms bonded tgther (carbon fibres) or from carbon nanotubes. These composites r v strong & light so r used in aerospace & sports car manufacturing
Concrete is made from aggregate (mixture of sand & gravel) embedded in cement. Its v strong, makes it ideal for use as building material (in skate parks)
wood is natural composite of cellulose fibres held tgther by organic polymer matrix
2 things that can influence properties of polymer, example
2 things can influence properties of a polymer - how its made & what its made from
properties of poly(ethene) depend on catalyst that was used & reaction conditions (temp & pressure) that i wad made under
Low density (LD) poly(ethene) made from ethene at moderate temp under high pressure. Its flexible & used for bags & bottles
High density (HD) poly(ethene) is made from ethene but at lower temp & pressure w catalyst. More rigid & used for water tanks & drainpipes
monomers a polymers made from determine…, 2 examples
monomers a polymer is made from determine type of bonds that form between polymer chains. These weak bonds between diff molecule chains determine properties of polymer:
thermosoftening polymers → contain individual polymer chains entwined tgther w weak forces between chains. Can melt these plastics & remould them
thermosetting polymers → contain monomers that can form cross-links between polymer chains, holding chains tgther in solid structure. Unlike thermosoftening polymers, these dont soften when heated, they r strong, hard & rigid
what do ceramics include & properties, polymers r… & have many applications…, properties of composites depend…, metals r… & uses
ceramics include glass & clay ceramics such as porcelain & bricks. They’re insulators of heat & electricity, brittle (arent flexible & break easily) & stiff
polymers r insulators of heat & electricity, can be flexible (bent w/out breaking) & r easily moulded. Polymers have many applications including in clothing & insulators in electrical items
properties of composites depend on matrix/ binder & reinforcement used to make them so they have many diff uses
metals r malleable, good conductors of heat & electricity, ductile (can be drawn into wires), shiny & stiff. Metals have many uses, including in electrical wires, car body-work, cutlery
regular structure of pure metals…, alloys r made…, example
regular structure of pure metals makes them soft - often too soft for use in everyday life
alloys r made by adding another element to metal. This disrupts structure of metal making alloys harder than pure metals
example → alloys of iron called steels r often used instead of pure iron. Steels r made by adding small amounts of carbon & sometimes other metals to iron
4 other alloys that r used in real life
Bronze = copper + tin: Bronze is harder than copper, its used to make medals, decorative ornaments & statues
Brass= copper + zinc: Brass is more malleable than bronze & is used in situations where low friction is required, such as in water taps & door fittings
Gold alloys r used to make jewellery: Pure gold is v soft, metals such as zinc, copper, silver r used harden gold. Pure gold is described as 24 carat, so 18 carats means 18/24 parts of alloy r gold. In other words 18 carat gold is 75%
aluminium alloys r used to make aircraft: aluminium has low density which is important property in aircraft manufacture. But pure aluminium is too soft for making aeroplanes so its alloyed w small amounts of other metals to make it stronger
iron corrodes easily in other words…, To rust iron needs.., equation, where does corrosion happen, what does rust do & this means, aluminium also.. & this is because
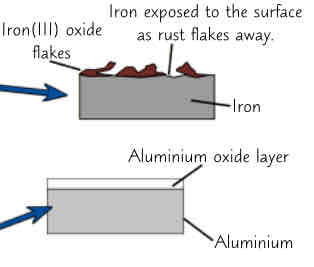
iron corrodes easily in other words it rusts (only iron)
To rust iron need to be in contact with oxygen & water which r present in air
iron + oxygen + water → hydrated iron (III) oxide
corrosions only happens on surface of material where its exposed to air
Rust is soft crumbly solid that soon flakes off to leave more iron available to rust again. This means eventually all iron in object corrodes away even if it wasn’t initially at surface
Aluminium also corrodes when exposed to air. Unlike iron objects, things made from aluminium arent completely destroyed by corrosion.Because aluminium oxide that forms when aluminium corrodes doesnt flake away, forms protective layer that sticks firmly to aluminium below & stops any further reaction taking place
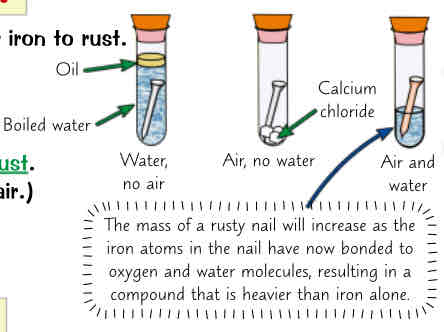
experiments which show that both oxygen & water r needed for iron to rust
if put iron nail in boiling tube w just water, it wont rust ( water is boiled to remove oxygen & oil is used to stop air getting in)
if put iron nail in boiling tube w just air it wont rust (calcium chloride can be used to absorb any water from air)
however if put iron nail in boiling tube w air & water it will rust
3 ways to prevent rusting by barrier methods & when r they used
painting/ coating w plastic → ideal for big & small structures alike. Can be decorative too
Electroplating → uses electrolysis to reduce metal ions onto an iron electrode. Can be used to coat iron w layer of diff metal that wont be corroded away
oiling/ greasing → has to be used when moving parts r involved like on bike chains
how to prevent rusting w sacrificial method, protective techniques (use both barrier & sacrificial)
sacrificial method → involves placing more reactive metal such as zinc/ magnesium w iron. Water & oxygen then react w sacrificial metal instead of iron
protection techniques → object can be galvanised by spraying it w coating of zinc. Zinc layer firstly protective but if its scratched the zinc around site of scratch works as a sacrificial metal
how do natural resources form & example, what can replace them & example, agriculture provides… & e.g.
natural resources form w/out human input. They include anything that comes from earth, sea, air. example → cotton for clothing or oil for fuel
some of natural products can be replaced by synthetic products or improved upon by man-made processes. Example → rubber is natural product that can be extracted from sap of a tree however man-made polymers have now been made which can replace rubber in uses such as tyres
agriculture provides conditions where natural resources can be enchanced for our needs. E.g. development of fertilisers have meant we can produce a high yield of crops
renewable resources reform…, example & other include, finite resources arent…, they include…, after they’ve been extracted… & example
renewable resources reform at similar rate to, or faster than we use them
example → timber is renewable resources as trees can be planted following harvest & only take few years to regrow. Other examples include fresh water & food
finite resources arent formed quickly enough to be considered replaceable
finite resources include fossil fuels & nuclear fuels such as uranium & plutonium. Minerals & metals found in ores in earth r also non-renewable materials
after they’ve been extracted many finite resources undergo man-made processes to provide fuels & materials necessary for modern life. Example → fractional distillation is used to produce usable products such as petrol from crude oil & metal ores r reduced to produce pure metal
many modern materials r made from…, peope have to balance…, example
many modern materials r made from raw, finite resources for example most plastics, metals & building materials
people have yo balance social, economic & environmental effects ot extracting finite resources
example —> mining metal ores is good because useful products can be made. Also provides local pple w jobs & brings money into area. However mining ores is bad for environment as uses loads energy, scars landscape, produces lots waste & destroys habitats
whats sustainable development, 3 things that r unsustainable to use, 1 way of reducing use of.., cant stop using finite resources altogether… & example
sustainable development is an approach to development that takes account of needs of present society while not damaging lives of future generations
not all resources r renewable so its unsustainable to keep using them & so is extracting resources due to amount of energy used & waste produced. Processing resources into useful materials such as glass/ bricks as process often used energy thats made from finite resources
1 way reducing use of finite resources is for pple to use less. Doesn’t just reduce use of that resource but also anything needed to produce it
cant stop using finite resources altogether but chemists can develop & adapt processes that use lower amounts of finite resources & reduce damage to environment. Example → chemists have developed catalysts that reduce amount of energy required for certain industrial processes
what copper & way of improving sustainability, 2 ways of doing this, traditional methods of copper mining…
copper is finite resource, one way to improve its sustainability is by extracting it from low-grade ores (ores w/out much copper in) scientists r looking into new ways of doing this:
biobleaching → bacteria r used to convert copper compounds in ore into soluble copper compounds, separating out copper from ore in process. The leachate (solution produced by process) contains copper ions, which can be extracted e.g. by electrolysis/ displacement w more reactive metal (scrap iron)
phytomining → involves growing plants in soil that contains copper. The plants cant use/ get rid of copper so it gradually builds up in leaves. The plants cant be harvested, dried & burned in furnace. The ash contains soluble copper compounds from which copper can be extracted by electrolysis/ displacement using scrap irons
traditional methods of copper mining r pretty damaging to environment. These new methods of extraction have a much smaller impact but disadvantage is that they’re slow
mining & extracting metals takes, recycling metals often uses much less energy…, metals r usually recycled by…, depending on what metals will be used for… & example
mining & extracting metals takes lots of energy, most of which comes from burning fossil fuels
recycling metals often uses much less energy than is needed to mine & extract new metals, conserves finite amount of each metal in earth & cuts down on amount of waste getting sent to landfill
metals r usually recycled by melting them & then casting them into shape of new product
depending on what metal will be used for after recycling the amount of separation required for recyclable metals can change. Example → waste steel & iron can be kept tgther as they can both be added to iron in blast furnace to reduce amount of iron ore required
glass recycling can help sustainability by…, glass bottles can often…, other forms of glass cant be…, glass is crushed and…
glass recycling can help sustainability by reducing amount of energy needed to make new glass products, & also amount of waste created when used glass is thrown away
glass bottles can often be reused w/out reshaping
other forms of glass cant be reused so they’re recycled instead. Usually glass is separated by colour & chemical compositions before being recycled
glass is crushed & melted to be reshaped for use in glass products such as bottles/ jars. might also be used for diff purpose such as insulating glass wool for wall insulation in homes
how to make life cycle assessments (4 steps) getting raw material(2), manufacture & packaging(2), using product(2), product disposal(3)
getting raw materials:
extracting raw materials needed for product can damage local environment (mining metals). Extraction can also result in pollution due to amount of energy needed
raw materials often need to be processed to extract desired materials & often needs large amount of energy (extracting metals from ores/ fractional distillation of crude oil
manufacturing & packaging:
manufacturing products & their packaging can use lots of energy resources & can also cause lot of pollution (harmful fumes such as carbon monoxide/ hydrogen chloride)
also need to think about any waste products & how to dispose of them. The chemical reactions used to make compounds from their raw materials can produce waste products. Some waste can be turned into other useful chemicals, reducing amount that ends up polluting environment
using the product:
use of a product can damage environment. Example → burning fuels releases greenhouse gases & other harmful substances. Fertilisers can leach into streams & rivers causing damage to ecosystems
how long a product is used for or how many uses it gets is also factor - products that need lots energy to produce but r used for ages mean less waste in long run
product disposal:
products r often disposed of in landfill sites. This takes up space & pollutes land & water (if paint washes off a product & gets into rivers)
energy is used to transport waste to landifll, which causes pollutants to be released into atmosphere
products might be incinerated (burnt), which causes air pollution
how can compare life cycle assessments for plastic & paper bags
…
life cycle assessment have shown even though plastic bags aren’t biodegradable they take less energy to make & have longer lifespan than paper bags so may be less harmful to environment

3 problems w life cycle assessments
use of energy, some natural resources & amount of certain types of waste produced by product over its lifetime can be easily quantified. But effect of some pollutants is harder to give a numerical number to E.g. its difficult to apply value to negative visual effects of plastic bags in environment compared to paper ones
producing an LCA (life cycle assessment) is not an objective method as it takes into account the values of person carrying out assessment. Means LCAs can be biased
Selective LCAs which only show some of impacts of product on the environment can also be biased as they can be written to deliberately support claims of a company in order to give them positive advertising
whats potable water, its not pure…, the important thing…
potable water → water thats been treated/ is naturally safe for humans to drink - essential for life
its not pure though. Pure water only contains H_2O molecules whereas potable water can contains lots of other dissolved substances
important things = levels of dissolved salts aren’t too high, that it has pH between 6.5 & 8.5 & also that there aren’t any bacteria/other microbes swimming around in it
rainwater is type… & what is it, when it rains water collects as…, source of fresh water depends on & surface water tends to…,
rainwater is type of fresh water → water that doesnt have much dissolved in it
when rains, water can either collect as surface water (in lakes, rivers, reservoirs) or as groundwater (in rocks called aquifers that trap water underground)
In UK source of fresh water depends on location. Surface water tends to dry up first so in warm areas (south-east most of domestic water supply comes from groundwater)
even if it only has low levels of dissolved substances…, 2 things this process includes
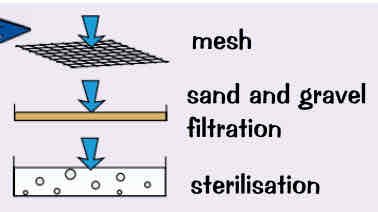
even if has low levels of dissolved substances, water from these fresh water sources still need to be treated to make it safe before it can be used. This process includes:
filtration → wire mesh screens out large twigs ect, & then gravel & sand beds filter out any other solid bits
sterilisation → water is sterilised to kill any harmful bacteria/ microbes. Can be done by bubbling chlorine gas through it or by using ozone or ultraviolet light
how provide potable water in very dry countries, how can test & distil water in lab (4)
in very dry countries (Kuwait) theres nit enough surface or groundwater & instead sea water must br treated by desalination to provide potable water
distillation can be used to desalinate sea water:
test pH of water using pH meter. If pH is too high/ too low can neutralise it. Do this with titration but use a pH meter to tell you when solutions neutral rather than an indicator as this wont contaminate water
should also test water for presence of sodium chloride (main salt in seawater). To test for sodium ions, do a flame test on small sample. If sodium ions r present the flame will turn yellow. To test for chloride ions, take another sample of ur water & add few drops of dilute nitric acid, followed by few drops of silver nitrate solution. If chloride ions r present, a white precipitate will form
to distil the water, pour the salty water into distillation apparatus. Heat flask from below. The water will boil & form steam, leaving any dissolved salts in flask. The steam will condense back to liquid water in condenser & can be collected as it runs out
Then, retest distilled water for sodium chloride to check that it has been removed. Also retest pH of water with a pH meter to check that its neutral (pH of 7)
what can sea water also be treated by, both distillation & reverse osmosis need…
sea water can also be treated by processes that use membranes - like reverse osmosis. The salty water is passed through membrane that only allows water molecules to pass through. Ions & larger molecules r trapped by membrane so separated from water
both of distillation & reverse osmosis need loads of energy, so they’re really expensive & not practical for producing large quantities of fresh water
what do we use water for at home, agricultural system also produce, sewage from domestic/agricultural sources have to… & other it would…, industrial processes such as…, as well as organic matter…
use water for lots things at home - having bath, going toilet, doing wash-up ect. When flush this water down drain, it goes into sewers & towards sewage treatment plants
agricultural system also produces a lot of waste water including nutrient run-off from fields & slurry from animal farms
sewage from domestic/ agricultural sources has to be treated to remove any organic matter & harmful microbes before it can be put back into freshwater sources like rivers/ lakes. Otherwise it would make them very polluted & would pose health risks
industrial processes such as haber process also produce lots of waste water that has to be controlled & treated
as well as organic matter, industrial waste water can also contain harmful chemicals - so it has to undergo additional stages of treatment before it is safe to release it into environment
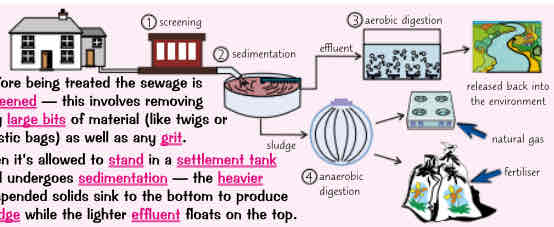
how does sewage treatment happen (6 steps), sewage treatment requires… & example
before being treated the sewage is screened - involves removing any large bits of material (twigs/ plastic bags) as well as any grit
then its allowed to stand in a settlement tank & undergoes sedimentation - the heavier suspended solids sink to bottom to produce sludge while lighter effluent floats on the top
the effluent in settlement tank is removed & treated by biological aerobic digestion. This is when air is pumped through water to encourage aerobic bacteria to break down any organic matter - including other microbes in water
the sludge from bottom of settlement tank is also removed & transferred into large tanks. Here it gets broken down by bacteria in process called anaerobic digestion
anaerobic digestion breaks down organic matter in sludge, releasing methane gas in process. The methane gas can be used as an energy source & remaining digested waste can be used as fertiliser
for waste water containing toxic substances, additional stages of treatment may involve adding chemicals (e.g. to precipitate metals), UV radiation or using membranes
sewage treatment requires more processes than treating fresh water but uses less energy than desalination of salt water, could be used as an alternative in areas where theres not much fresh water. Example → Singapore is treating waste water & recycling it back into drinking supplies. However, pple dont like idea of drinking water that used to be sewage
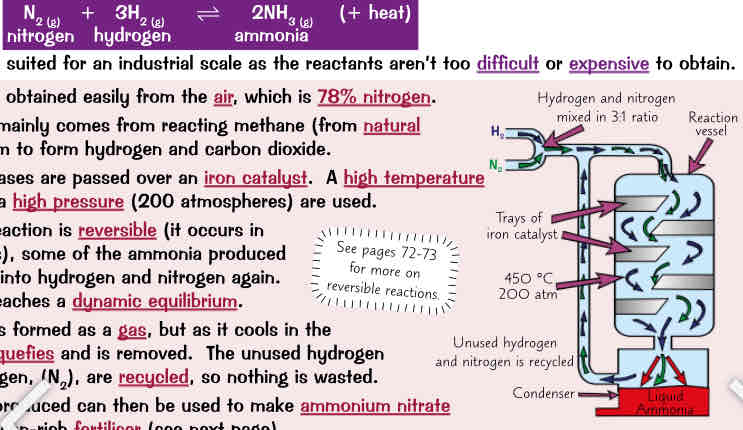
Haber process reaction, this process…, how make ammonia using this reaction (6)
This reaction is well suited for industrial scale as reactants arent too difficult or expensive to obtain:
nitrogen is obtained easily from air which is 78% nitrogen
hydrogen mainly comes from reacting methane (from natural gas) w steam to form hydrogen & carbon dioxide
reactant gases r passed over an iron catalyst. A high temp (450°) & high pressure (200 atmospheres) r used
because reaction is reversible (occurs in both directions) some of ammonia produced converts back into hydrogen & nitrogen again. It eventually reaches a dynamic equilibrium
ammonia is formed as gas but as it cools in the condenser it liquefies & is removed. The unused hydrogen (H_2) & nitrogen (N_2) r recycled so nothing is wasted
the ammonia produced can then be used to make ammonium nitrate - very nitrogen-rich fertiliser
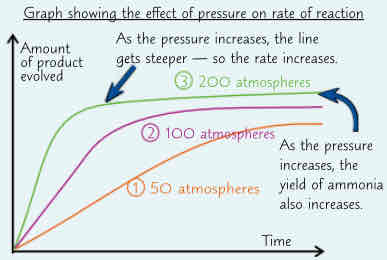
these factors can…, example, problem, whats the compromise, higher pressures move…, pressure is set…, iron catalyst makes…
these factors (temp, pressure) can also affect position of equilibrium for reversible reaction - & sometimes there is trade-off between increasing rate & maximising yield
example → forward reaction in Haber process is exothermic. Means that increasing temp will move equilibrium the wrong way - away from ammonia & towards nitrogen & hydrogen. So yield of ammonia would be greater at lower temps
problem → lower temps mean slower rate of reaction ( & equilibrium is reached more slowly)
450° is a compromise between maximum yield & speed of reaction. Better wait 20s for 10% yield than have to wait 60s for 20% yield
higher pressures move position of equilibrium towards products since there r 4 molecules of gas on left-hand side for every 2 molecules on the right. So increasing pressure maximises the percentage yield. Also increases rate of reaction
pressure is set as high as possible w/out making process too expensive/ dangerous to build & maintain. Hence 200 atmospheres operating pressure
iron catalyst makes reaction go faster but doesnt affect yield
what did farmers use to fertilise & whats better & why, 3 main essentials in fertiliser & if plant dont get it & may go missing…, what do they replace & example, whats NPK fertilisers
farmers used manure to fertilise fields. Formulated fertilisers r better as they’re more widely available, easier to use, done smell & have just enough of each nutrient so more crops can be grown
3 main essential elements in fertilisers r nitrogen, phosphorus, potassium. If plants dont get enough of these elements their growth & life processes r affected. These elements may be missing from soil if they’ve been used up by previous crop
fertilisers replace these missing element/ provide more of them. Helps increase crop yield, as crops can grown faster & bigger. Example → fertilisers add more nitrogen to plant proteins, which makes plants grow faster - increasing productivity
NPK fertilisers r formulations containing salts of nitrogen (N), phosphorus (P), potassium (K) in right percentages of elements
what can ammonia react w, how to get ammonium salts, equation for ammonium nitrate, ammonia & nitrate react tgther…
ammonia can be reacted w oxygen & water in series of reactions to make nitric acid
can also react ammonia w acids, including nitric acid to get ammonium salts
Ammonia & nitric acid react tgther to produce ammonium nitrate - especially good compound to use in fertiliser because has nitrogen from 2 sources
hows ammonium nitrate reaction carries out in industry, in lab?
industry → reaction is carried out in giant vents, at high concentrations resulting in very exothermic reaction. Heat released is used to evaporate water from mixture to make very concentrated ammonium nitrate product
in lab → reaction is carried out on much smaller scale by titration & crystallisation. Reactants r at much lower concentration than in industry so less heat is produced by reaction & its safer for person to carry it out. After titration, the mixture need to be crystallised to give pure ammonium nitrate crystals. Crystallisation isnt used in industry because its very slow
what can be mined as source of potassium, what cant plants use as nutrients, 3 phosphate rock reactions w no. diff types acids produce soluble phosphates (3)
potassium chloride & potassium sulphate can be mined & used as source of potassium
phosphate rock is also mined but because phosphate salts in rock r insoluble plants cant use them as nutrients
reacting phosphate rock w no. diff types of acids produces soluble phosphates:
reaction w nitric acid produces phosphoric acid & calcium nitrate
reaction w sulfuric acid produces calcium sulfate & calcium phosphate (mixture known as single superphosphate)
reaction w phosphoric acid only produces calcium phosphate (product of reaction can be called triple superphosphate )