Medicinal Chemistry of Acetylcholinesterase Inhibitors and Alzheimer’s Treatments
1/205
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
206 Terms
Acetylcholinesterase (AChE)
An enzyme that promotes acetylcholine hydrolysis by forming an intermediate (acetyl-AChE) with the release of choline.
Hydrolysis of acetylcholine
The process where acetylcholine is broken down into acetate and choline by acetylcholinesterase.
Efficiency of AChE
Each molecule of AChE can hydrolyze 25,000 molecules of acetylcholine each second.
Active site of AChE
Located at the base of a narrow gorge about 20 angstroms deep, surrounded by 14 aromatic amino acid residues.
Anti-ChEs
Drugs that inhibit AChE, also referred to as cholinesterase (ChE) inhibitors, which inhibit both AChE and BChE.
Butyrylcholinesterase (BChE)
An enzyme found in liver and plasma that metabolizes circulating esters, not abundant in nerve ending synapses.
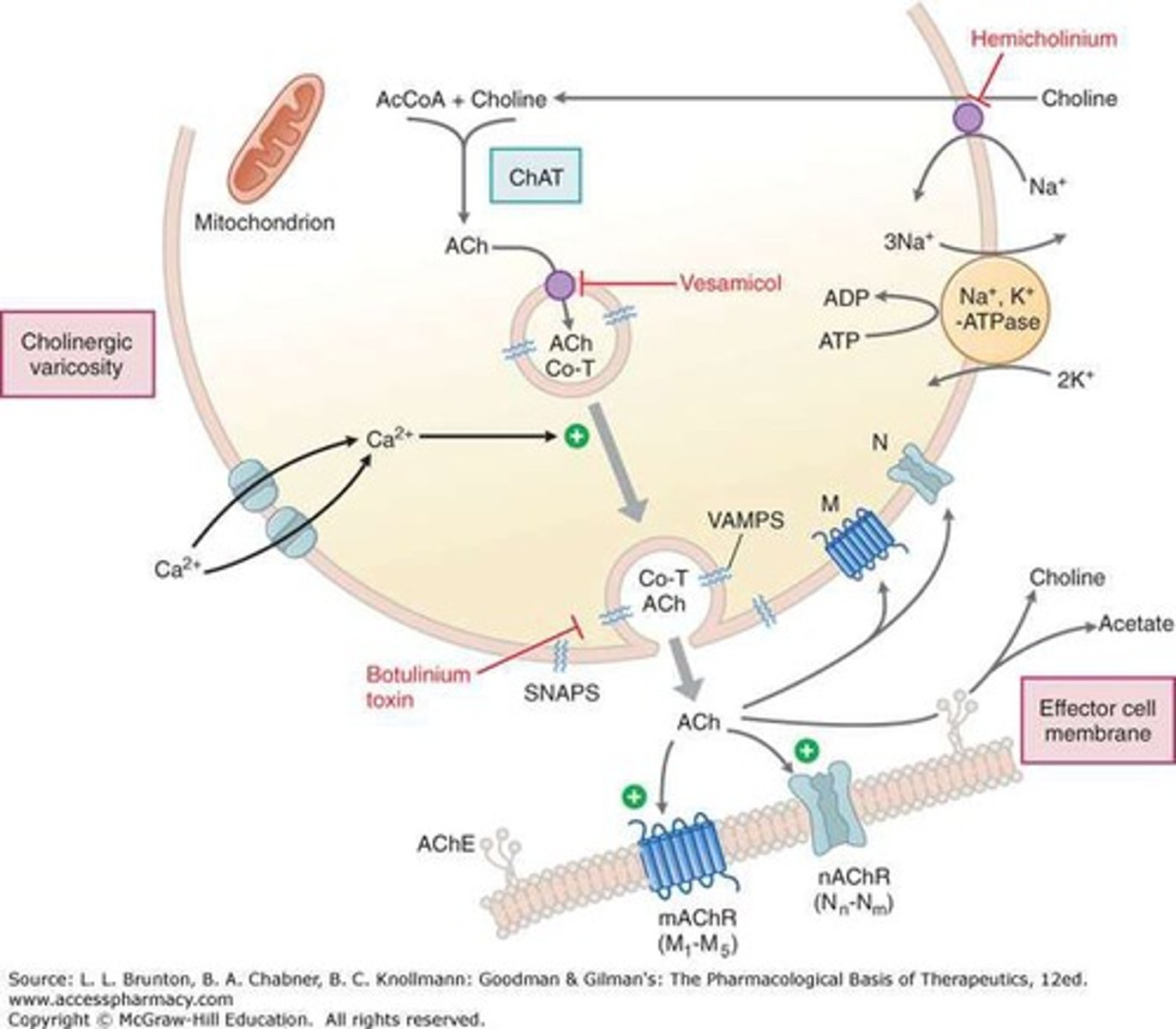
Accumulation of ACh
AChE inhibitors cause acetylcholine to accumulate in the vicinity of cholinergic nerve terminals, potentially producing excessive stimulation of cholinergic receptors.
Neurotransmitter inactivation routes
Three major routes exist: neuronal reuptake, enzymatic inactivation, and diffusion into/reuptake via surrounding cells.
Choline acetyltransferase
An enzyme involved in the synthesis of acetylcholine from acetyl-CoA and choline.
Enzymatic inactivation
A process where neurotransmitters are hydrolyzed by various peptidases or diffuse out of the synapse.
Diffusion of neurotransmitters
Neurotransmitters naturally diffuse away from the synapse and can diffuse into surrounding cells, often glial cells.
Crystal structure of TcAChE
The crystal structure of Torpedo californica acetylcholinesterase was solved in 1991, belonging to the class of α/β proteins.
Structure of TcAChE
Consists of a 12-stranded central mixed β-sheet surrounded by 14 α-helices.
Catalytic triad of AChE
Includes the side chains of Glu334, His447, and Ser203, which are involved in the enzyme's catalytic activity.
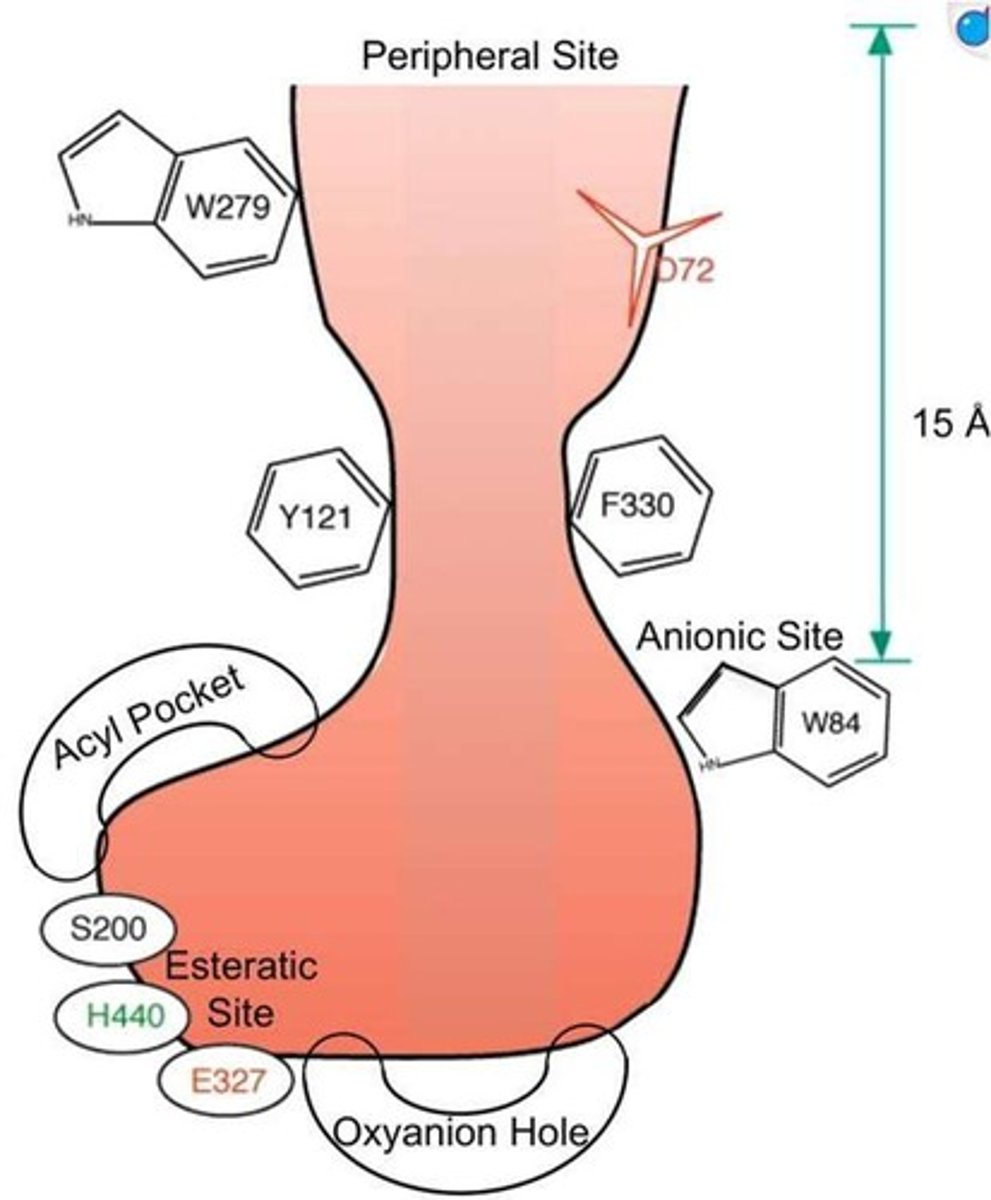
Acyl pocket of AChE
Composed of Phe295 and Phe297, it plays a role in the binding of the substrate.
Choline subsite of AChE
Includes Trp86, Glu202, and Tyr337, which are important for the binding of choline.
Peripheral site of AChE
Includes Trp286, Tyr72, Tyr124, and Asp74, which may influence the enzyme's activity.
Acetylcholinesterase
Exists in two general classes of molecular forms: simple homomeric oligomers of catalytic subunits (monomers, dimers, and tetramers) and heteromeric associations of catalytic subunits with structural subunits.
Homomeric forms of Acetylcholinesterase
Found as soluble species in the cell, presumably destined for export or for association with the outer membrane of the cell, typically through an attached glycophospholipid.
Heteromeric form of Acetylcholinesterase
Found mainly in neuronal synapses, is a tetramer of catalytic subunits disulfide-linked to a 20-kDa lipid-linked subunit and localized to the outer surface of the cell membrane.
Active site attraction
The positively charged acetylcholine is ionically attracted to the enzyme's active site by glutamic acid residue.
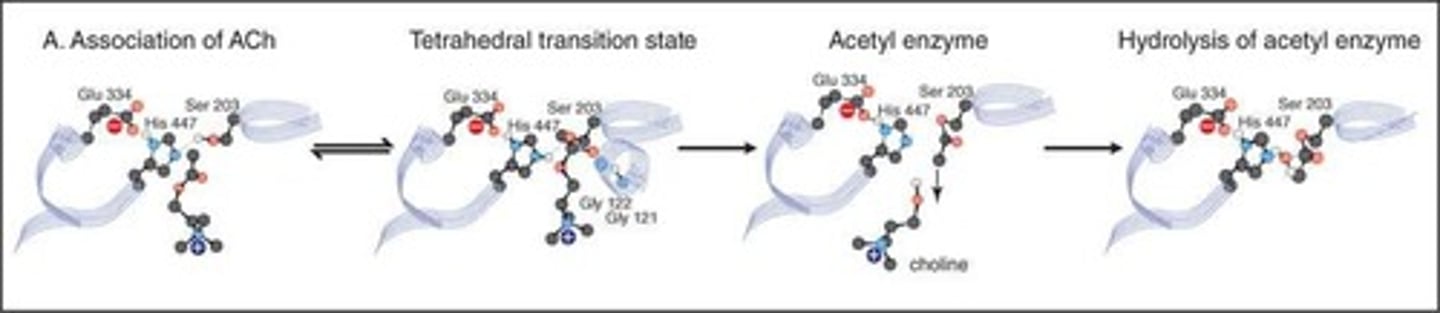
Catalytic triad mechanism
At the catalytic triad site, the basic imidazole ring of a His is protonated by a nearby Glu, making the oxygen atom of the hydroxyl group of Serine increasingly nucleophilic to attack the delta positive carbonyl in acetylcholine.
Tetrahedral transition state
The nucleophilic oxygen binds to the increasingly electrophilic (positively charged) carbonyl carbon to form a tetrahedral transition state intermediate.
Choline dissociation
Choline dissociates from the active site of AChE.
Acetyl group cleavage
The acetyl group on serine-203 is rapidly cleaved by water (rendered nucleophilic by a similar acid base enzymatic mechanism), to give the free functional enzyme again.
Active site structure
The active site of the enzyme lies at the base of a narrow gorge about 20 angstroms deep, surrounded by 14 aromatic amino acid residues.
Enzymatic hydrolases mechanism
Similar mechanisms involving the formation of negatively charged (O-) amino acid side chains in serine and threonine which then attack the carbonyl carbon of esters and amides are believed to function in many other hydrolytic enzymes.
Common proteases
These include common proteases such as trypsin, chymotrypsin, elastase and some enzymes involved in the formation of clotting factors, which all form similar catalytic triads.
Indirect acting cholinergics
Drugs that act by mimicking the effect of acetylcholine.
Direct cholinergic agents
Activate the muscarinic and/or nicotinic receptors.
Indirect cholinergic agents
Block acetylcholinesterase.
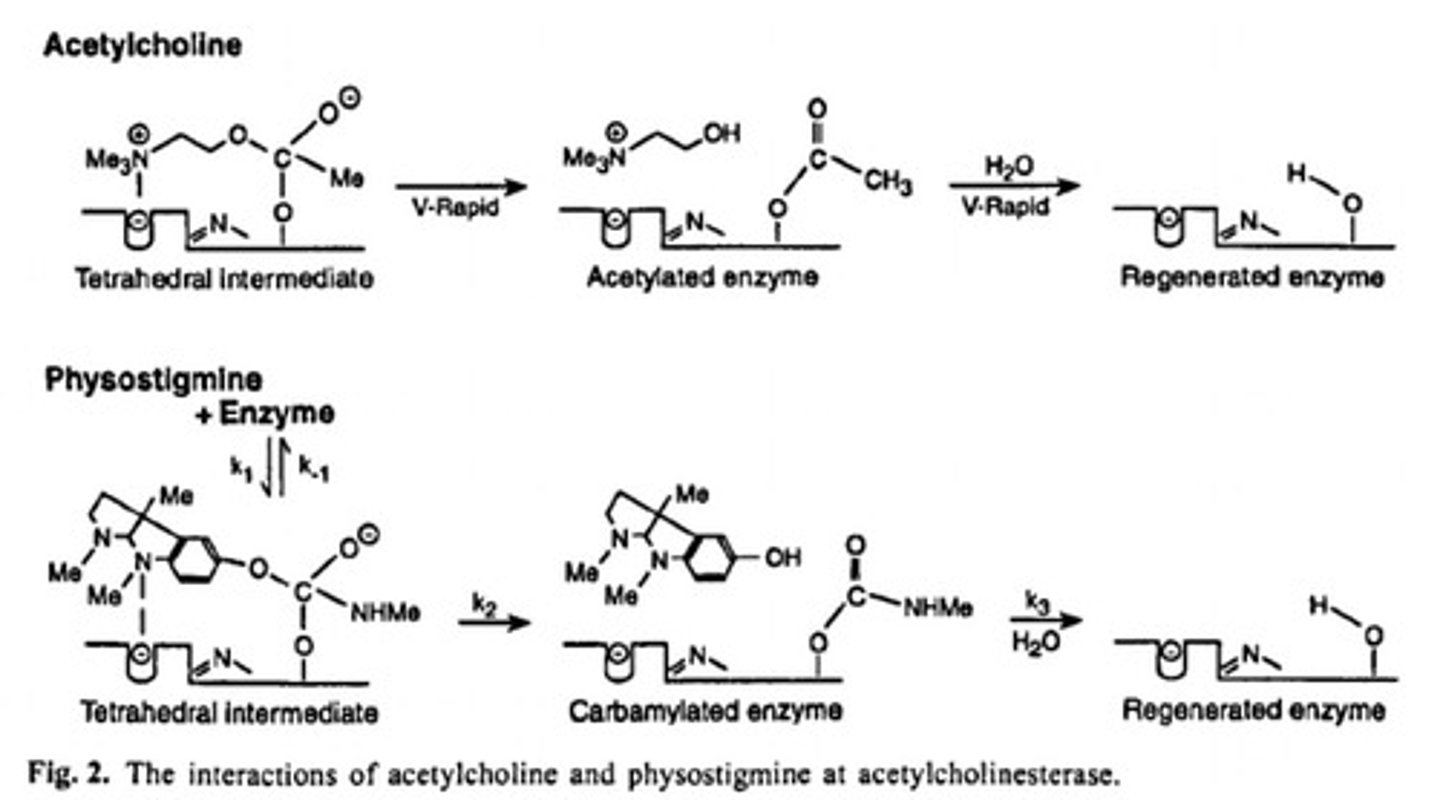
Aromatic carbamates
Form carbamoylated serine intermediate (Reversible) which inhibits the enzyme.
Organophosphates
Form phosphorylated serine intermediate (Irreversible) which inhibits the enzyme.
Physostigmine
Also called eserine, is an alkaloid obtained from the Calabar or ordeal bean, the dried, ripe seed of Physostigma venenosum, a perennial plant found in tropical West Africa.
Calabar bean usage
Once used by native tribes of West Africa as an 'ordeal poison' in trials for witchcraft, where guilt was judged by death from the poison and innocence by survival after ingestion of a bean.
Neostigmine bromide
More chemically stable than physostigmine with a longer duration of action. It does not cross the blood-brain barrier and is indicated for the reversal of non-depolarizing neuromuscular blocking agents after surgery and for treatment of Myasthenia gravis.
Pyridostigmine bromide
Indicated for the treatment of Myasthenia gravis and for reversal of neuromuscular blocking effects of nondepolarizing muscle relaxants. It has been used as a prophylactic agent against irreversible organophosphorus acetylcholinesterase inhibitors.
Rivastigmine tartrate
A parasympathomimetic or cholinergic reversible agent for the treatment of mild to moderate dementia of the Alzheimer's type, with FDA-approved transdermal patches for continuous drug delivery.
Cholinergics (Parasympathetics)
A class of drugs that mimic the action of acetylcholine in the body, often used in various therapeutic contexts.
Cholinesterase inhibitor
A substance that inhibits the enzyme cholinesterase, leading to increased levels of acetylcholine in the synaptic cleft.
Myasthenia gravis
An autoimmune disorder that leads to weakness in the skeletal muscles, often treated with cholinergic drugs.
FDA approval
The process by which the U.S. Food and Drug Administration evaluates and approves drugs for safety and efficacy before they can be marketed.
Transdermal patches
A method of drug delivery that allows medication to be absorbed through the skin into the bloodstream.
Anticholinergic toxicity
A condition resulting from the excessive blockade of acetylcholine receptors, often treated with cholinesterase inhibitors.
Soman
A potent nerve agent that inhibits acetylcholinesterase, leading to an accumulation of acetylcholine and potential respiratory failure.
Neurotransmitter
A chemical messenger that transmits signals across a chemical synapse from one neuron to another or to a target cell.
CNS
Central Nervous System, comprising the brain and spinal cord, responsible for processing information and coordinating activity.
Ester hydrolysis
A chemical reaction that breaks down esters into an acid and an alcohol, often affecting drug stability.
Dimethyl system
A chemical structure that provides steric protection from ester hydrolysis in certain cholinergic drugs.
Cholinergic crisis
A condition caused by excessive stimulation of the cholinergic system, often requiring medical intervention.
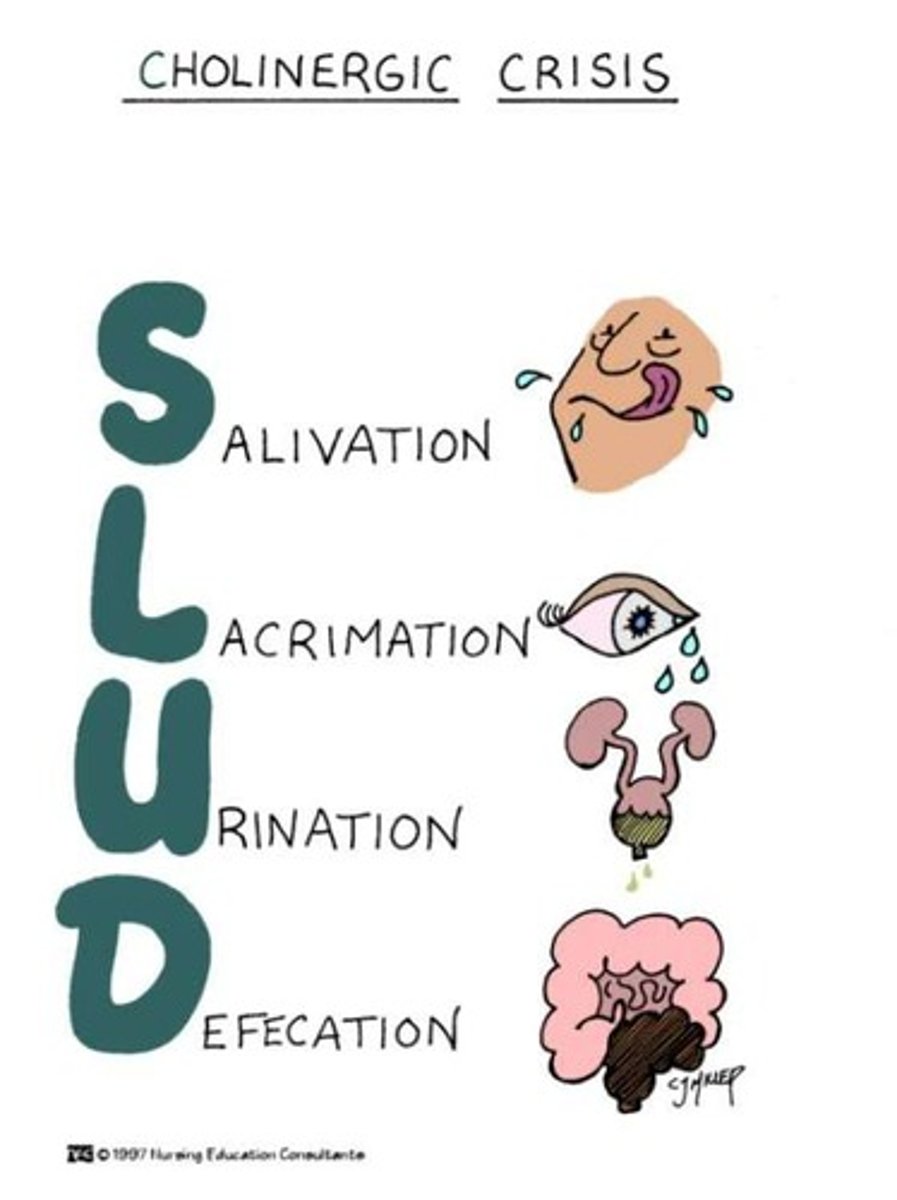
Neuromuscular blocking agents
Drugs that cause temporary paralysis by blocking the transmission of nerve impulses to the muscles.
Glaucoma
A group of eye conditions that damage the optic nerve, often associated with increased intraocular pressure.
Demecarium (Humorsol®)
This molecule is a dimer of neostigmine. In the eye, this causes constriction of the iris sphincter muscle (causing miosis) and the ciliary muscle. The outflow of the aqueous humor is facilitated, which leads to a reduction in intraocular pressure. Indicate Miotic agent (used post-operatively for cataract surgery).
Ambenonium chloride (Mytelase®)
The length of the 'linker' is important. This most likely allows the molecule to associate with two esterase units that are in proximity. This gives a slight increase in potency. It targets both muscarinic and nicotinic receptors. Indicate for Myasthenia gravis. US sales discontinued in 2012.
Edrophonium (Enlon®; Enlon-Plus®)
It is a very simple analog of neostigmine with an ethyl group. It shows short duration acting and less potency comparing with Neostigmine.
Current use of anti-AChE agents
Current use of anti-AChE agents is limited to four conditions in the periphery: Atony of the smooth muscle of the intestinal tract and urinary bladder, Glaucoma, via drop instillation into the eye, Myasthenia gravis, via parenteral and oral therapy, Reversal of the paralysis of competitive neuromuscular blocking drugs, via parenteral routes.
Cholinergics clinically utilized
Indirect acting Neurological diseases include Alzheimer's disease.
Tacrine (Cognex®)
A drug used in the treatment of Alzheimer's disease.
Donepezil (Aricept®)
A drug used in the treatment of Alzheimer's disease.
Galantamine (Razadyne®)
A drug used in the treatment of Alzheimer's disease.
Indirect acting-Irreversible inhibitors
Two groups of compounds can form much more stable intermediates than the acetylated serine, therefore, inhibit the enzyme: Aromatic carbamates-carbamoylated serine intermediate and Organophosphates-phosphorylated serine intermediate.
Nerve poisons
Some Phosphate Esters are capable of this chemical reaction. These agents are nerve poisons and are extremely toxic.
Phillippe de Clermont
1833, Jules Pelouze synthesized phosphoric acid monoethyl ether.
Anton Voegeli
1848, in Gustav Magnus lab, synthesized diethyl phosphonate and triethyl phosphonate.
Tetraethyl pyrophosphate
1854, Phillippe de Clermont synthesized tetraethyl pyrophosphate.
Dimethylamine-ethoxy-phosphorylcyanide
1903, Schall and Michaelis synthesized dimethylamine-ethoxy-phosphorylcyanide.
Jules Pelouze
Synthesized phosphoric acid monoethyl ether in 1833.
Schall and Michaelis
Synthesized dimethylamine-ethoxy-phosphorylcyanide in 1903.
Gerda von Krueger
Described the effects of inhaling organophosphates, including difficulty in breathing and blurred vision.
Sarin
An organophosphate that is one of the most toxic classes of agents known to man.
Chemical Warfare
Use of organophosphates to develop nerve gases during WWII.
Guanitoxin
A type of organophosphate.
Effects of Cholinergics
Include increased urination, blue skin, sweating, diarrhea, nausea, vomiting, difficulty breathing, and convulsions.
Organophosphate Structure
Tetrahedral in conformation, allowing them to bind to the active site more readily.
Enzyme Binding
Enzymes bind their substrates most strongly in the transition state.
Transition State Mimicry
The tetrahedral shape of organophosphates resembles the tetrahedral transition state of acetylcholine.
Penicillin
Resembles the transition state of the peptidoglycan substrate, making it a potent inhibitor of the glycopeptide transpeptidase enzyme.
Symptoms of Organophosphate Exposure
Include headache, anxiety, dizziness, coma, confusion, agitation, loss of appetite, and abdominal cramps.
Small Pupils
Pupils that are not reactive to light, a symptom of cholinergic toxicity.
Increased Tearing
A symptom associated with cholinergic effects.
Increased Salivation
A symptom associated with cholinergic effects.
Low or High Blood Pressure
A cardiovascular symptom of cholinergic toxicity.
Slow or Rapid Heart Rate
A cardiovascular symptom of cholinergic toxicity.
Weakness
A symptom associated with cholinergic toxicity.
Convulsions
A severe symptom of cholinergic toxicity.
Phosphorylated intermediate
The leaving group departs as a stabilized anion, resulting in a phosphorylated intermediate and an essentially irreversible inhibition of the enzyme that can last two weeks or more.
Diisopropyl phosphoryl enzyme
Formation of the diisopropyl phosphoryl (DFP) enzyme, formation of the aged monoisopropyl phosphoryl enzyme. Hydrolysis of the diisopropyl enzyme is very slow.
Aged monoisopropyl phosphoryl enzyme
The aged monoisopropyl phosphoryl enzyme is virtually resistant to hydrolysis and reactivation.
Amide bond hydrogens
Amide bond hydrogens from Gly121 and Gly122 stabilize the carbonyl and phosphoryl oxygens.
Isoflurophate (Floropryl®)
Indicated for topical use in the eye to treat certain types of glaucoma and other eye conditions, such as accommodative esotropia. Easily absorbed through skin.
Echothiophate Iodide (Phospholine®)
Indicated in the treatment of subacute or chronic angle-closure glaucoma after iridectomy or where surgery is refused or contraindicated.
Quaternary group
May ionically direct the compound into the active site, and it also serves to limit distribution beyond the eye.
Organophosphate poisoning
There are approximately one million organophosphate poisoning annually worldwide resulting in several hundred thousand deaths.
Aging process
Organophosphate poisoning is especially troublesome when the phosphorylated serine residues have undergone a process called 'aging'.
Pesticide usage in the U.S.
Approximately 1.1 billion pounds of pesticide active ingredients are used in the U.S. each year.
Insecticidal organophosphates
Many carbamates and lipophilic phosphate esters with high vapor pressures are used as insecticides.
Absorption of organophosphates
These compounds, especially the organophosphates, are readily absorbed via inhalation and transdermal through the skin.
Margin of safety
Compared to other organophosphate, it has a better margin of safety, because of different rates of metabolism in humans and in insects.
Hydrolysis of diisopropyl enzyme
Hydrolysis of the diisopropyl enzyme is very slow.
Cholinesterase levels
Echothiophate iodide for ophthalmic solution will depress both plasma and erythrocyte cholinesterase levels in most patients after a few weeks of eyedrop therapy.