Lecture 10 Carbohydrates
1/101
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
102 Terms
Carbohydrates
Aldehydes or ketones with at least two hydroxyl groups
-or substances that yield such compounds upon hydrolysis
-Empirical formula (CH2O)n
-Important components of key biomolecular structures (DNA, RNA, ATP, FAD)
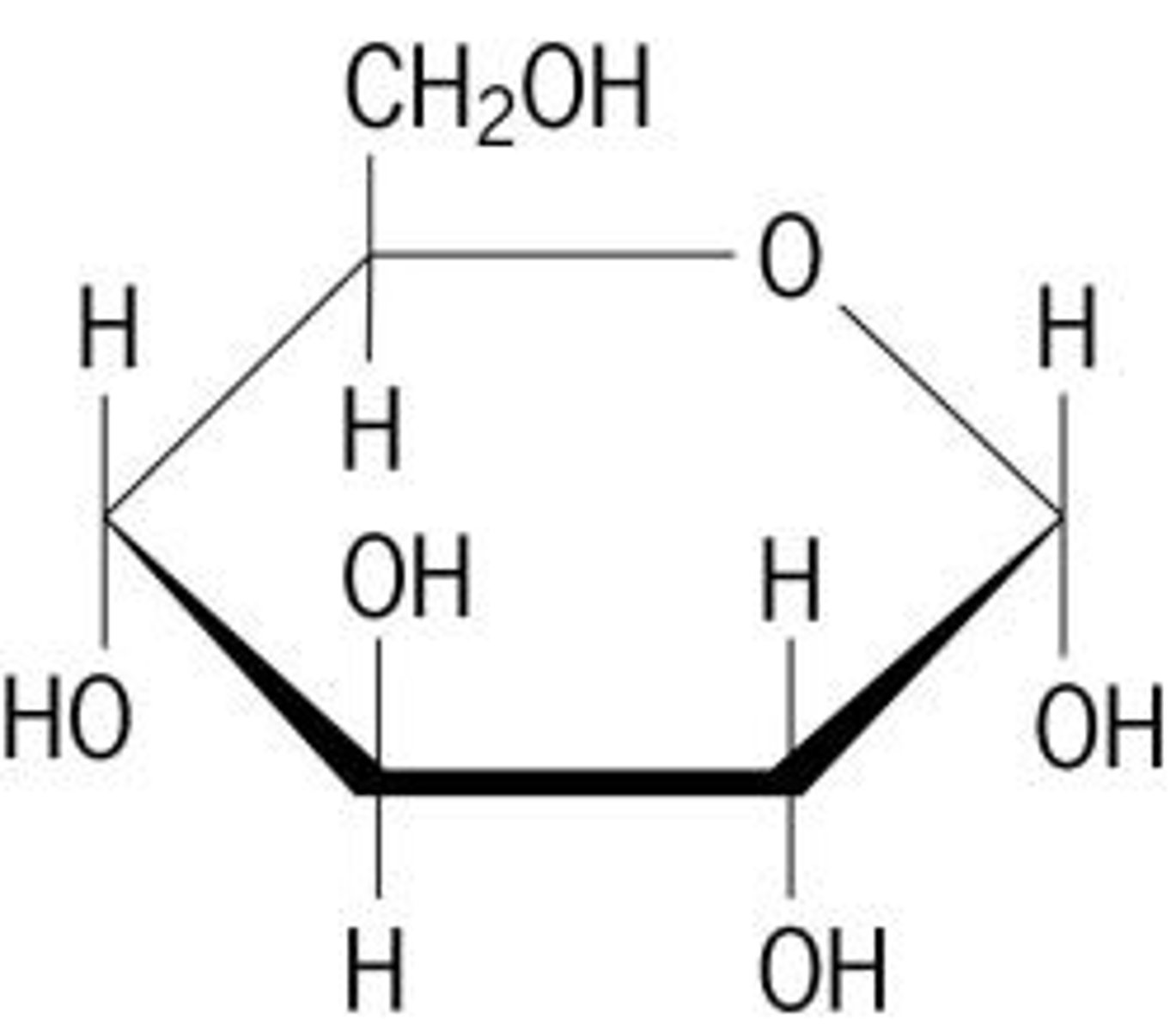
Monosaccharides
the building blocks of carbohydrate polymers.
-The specific sugar, linking pattern, and branching determine its function.
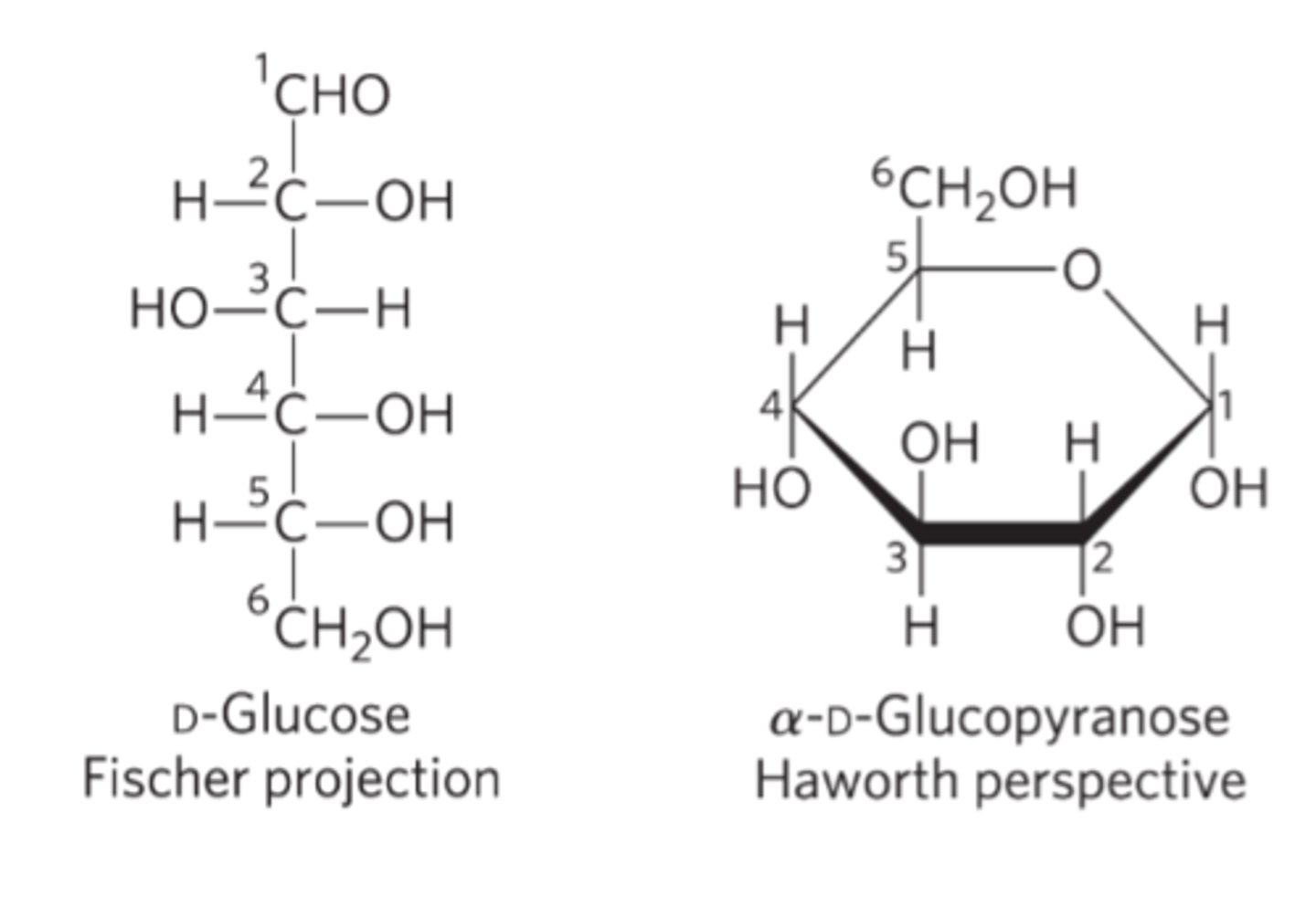
Fischer projection formulas
Used to represent 3D sugar structures on paper
-Bonds drawn horizontally indicate bonds that project up and out of the plane of the paper
-Bonds drawn vertically project behind the plane of the paper.
Monosaccharide structure
A single sugar unit.
-Link together to form more complex carbohydrates
-Cannot be broken down into simpler sugars, thus they are the most basic unit of carbohydrates
-They exist in either a straight-chain or cyclic structure when dissolved in water
-They must contain a carbonyl group (aldehyde or ketone) and multiple hydroxyl groups
-Classified as either aldoses (if aldehyde) or ketoses (if ketone)
Energy
monosaccharides (especially glucose) are used as a primary energy source in cellular respiration
Building blocks
Link to make larger carbohydrates
-Starch, cellulose, glycogen, and part of DNA/RNA
Biological structures
These are commonly used to form cellular structures
Signaling and Recognition
Can be part of glycoproteins and glycolipids on cell surface
-Important in cell-to-cell communication and immune responses.
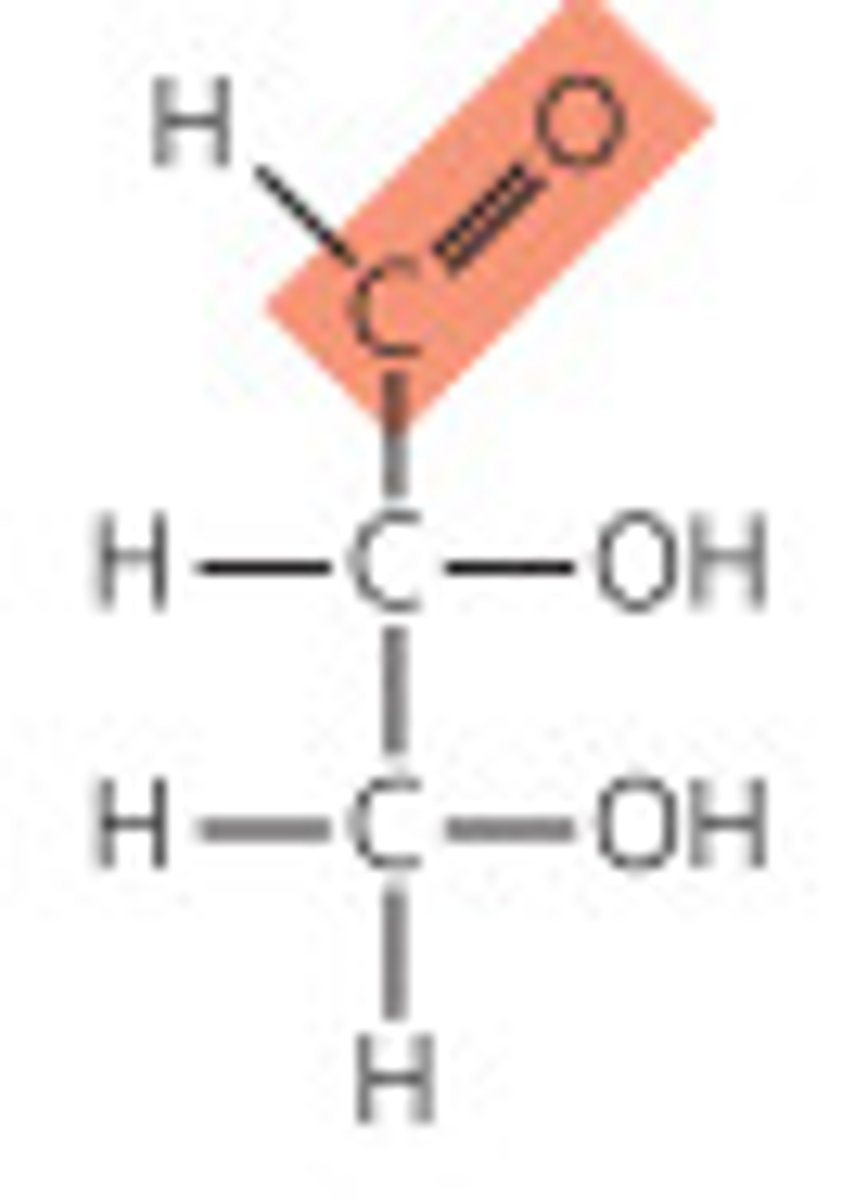
Aldose
carbonyl group is at an end of the carbon chain (in an aldehyde group)
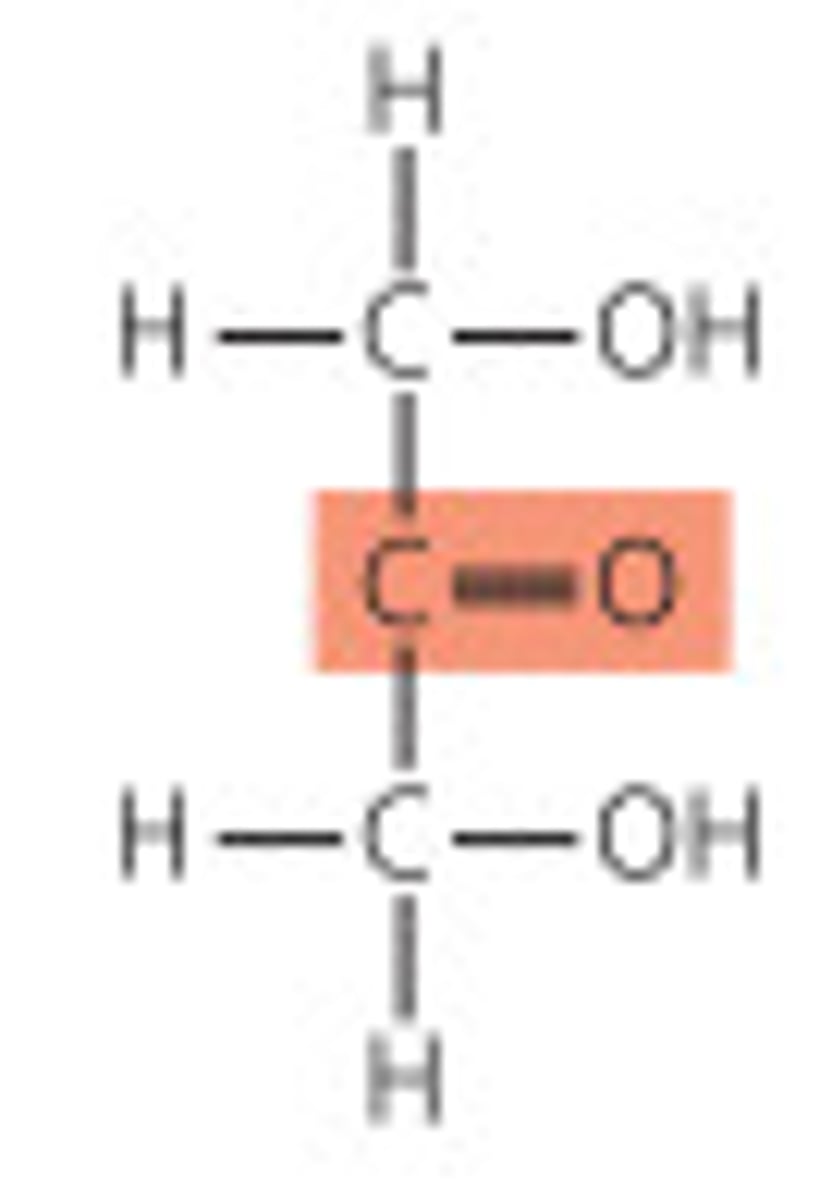
Ketose
carbonyl group is at any other position (in a ketone group)
Trioses
three carbon backbone
-The simplest monosaccharides
Tetroses
four carbon backbone
Pentoses
five carbon backbone
-Components of DNA and RNA
Hexoses
six carbon backbone
Heptoses
seven carbon backbone
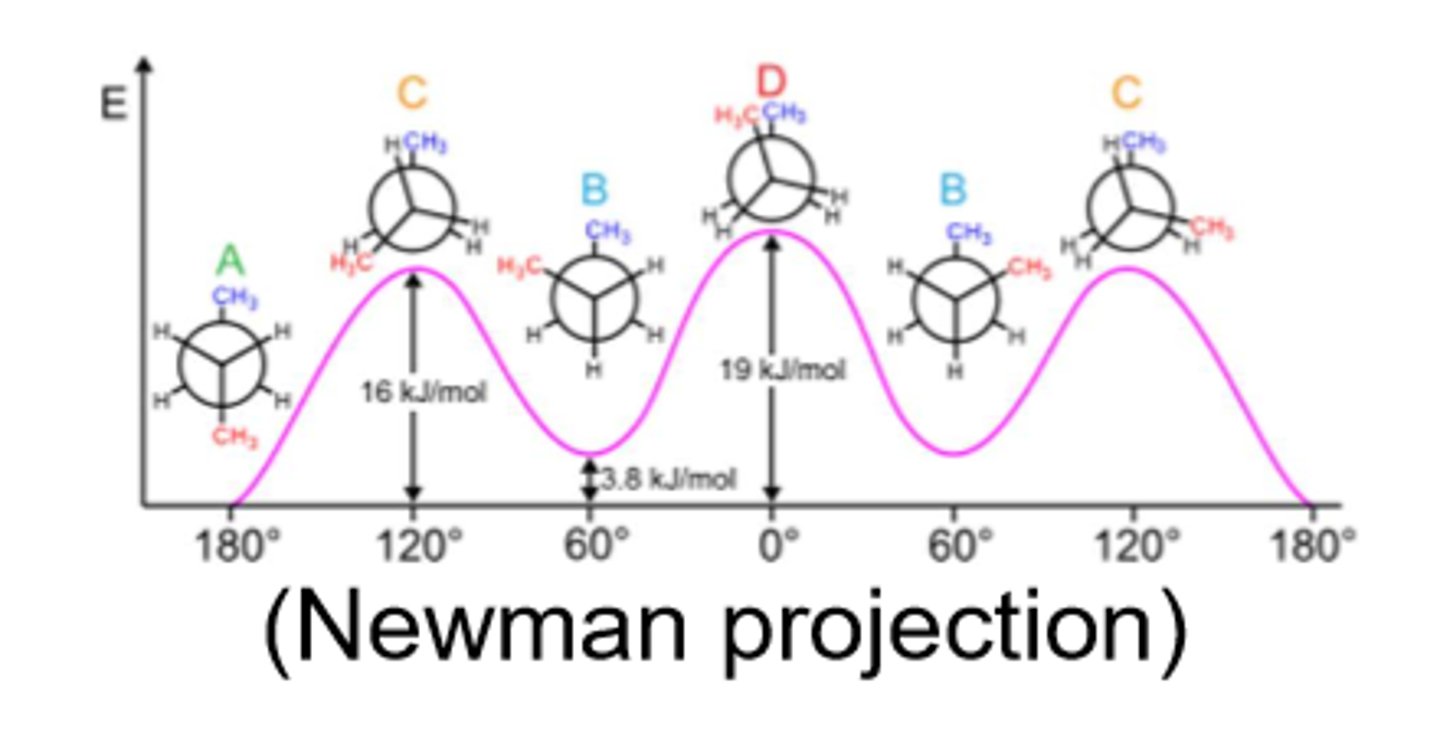
Stereoisomerism in sugars
In saccharides, many of the carbon atoms attached to hydroxyl groups are chiral centers.
-This is important because enzymes that act on sugars are stereospecific.
-Carbohydrates can have multiple chiral carbons.
-Stereochemistry determines interactions with other molecules
Biological carbohydrates
D conformation
Enantiomers
mirrored isomers
-Optically active
-Monosaccharides have >1 chiral carbons
-n chiral centers means 2^n stereoisomers
Reference carbon
the chiral center furthest from the carbonyl carbon
D isomers
OG on the reference carbon is on the right (dextro) in a projection formula
-In living organism
"LA to DC" (L amino acids and D carbohydrates are found in real life)
L isomers
OH on the reference carbon is on the left (levo) in a projection formula
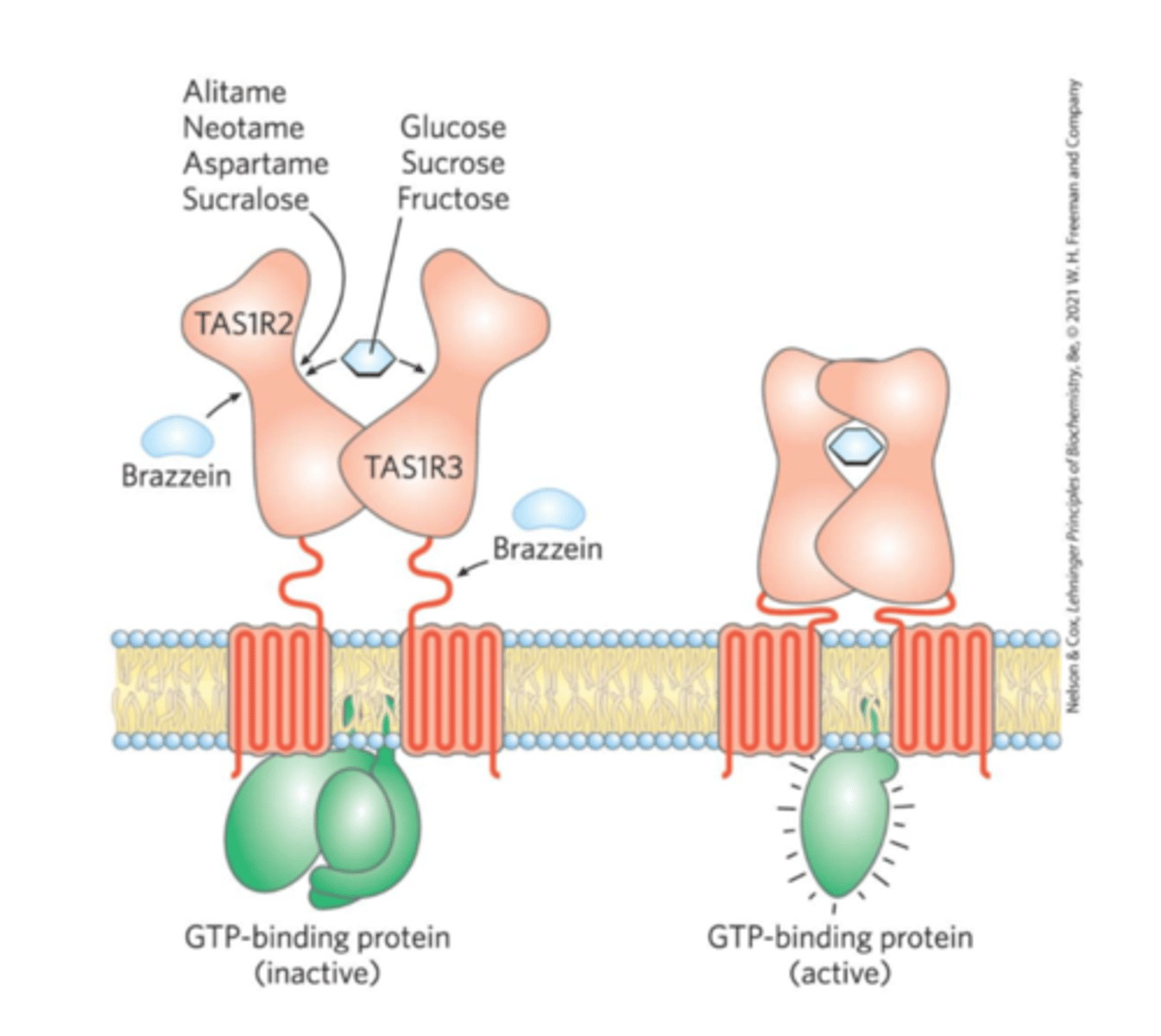
sweet-taste receptors
TAS1R2 and TAS1R3
-Binding of a compatible molecule generates a "sweet" electrical signal to the brain
-Requires a specific steric match
-Artificial sweeteners match the receptor but are not metabolized like normal sugars
numbering carbons of a sugar
carbons are numbered beginning at the end of the chain near the carbonyl group
-This is not for reference carbons in identifying isomers
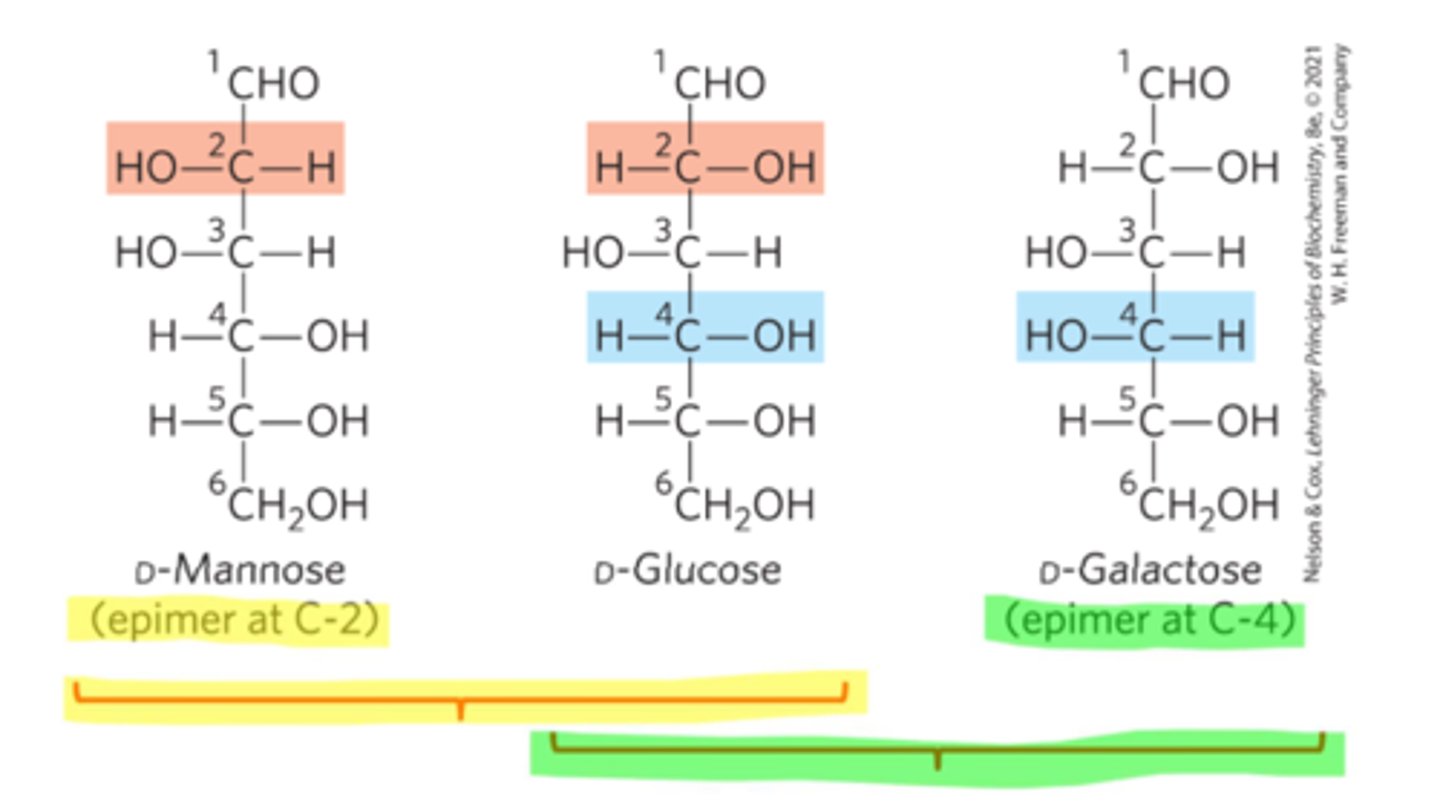
Epimers
Stereoisomers that have >1 chiral carbons, but only differ at 1
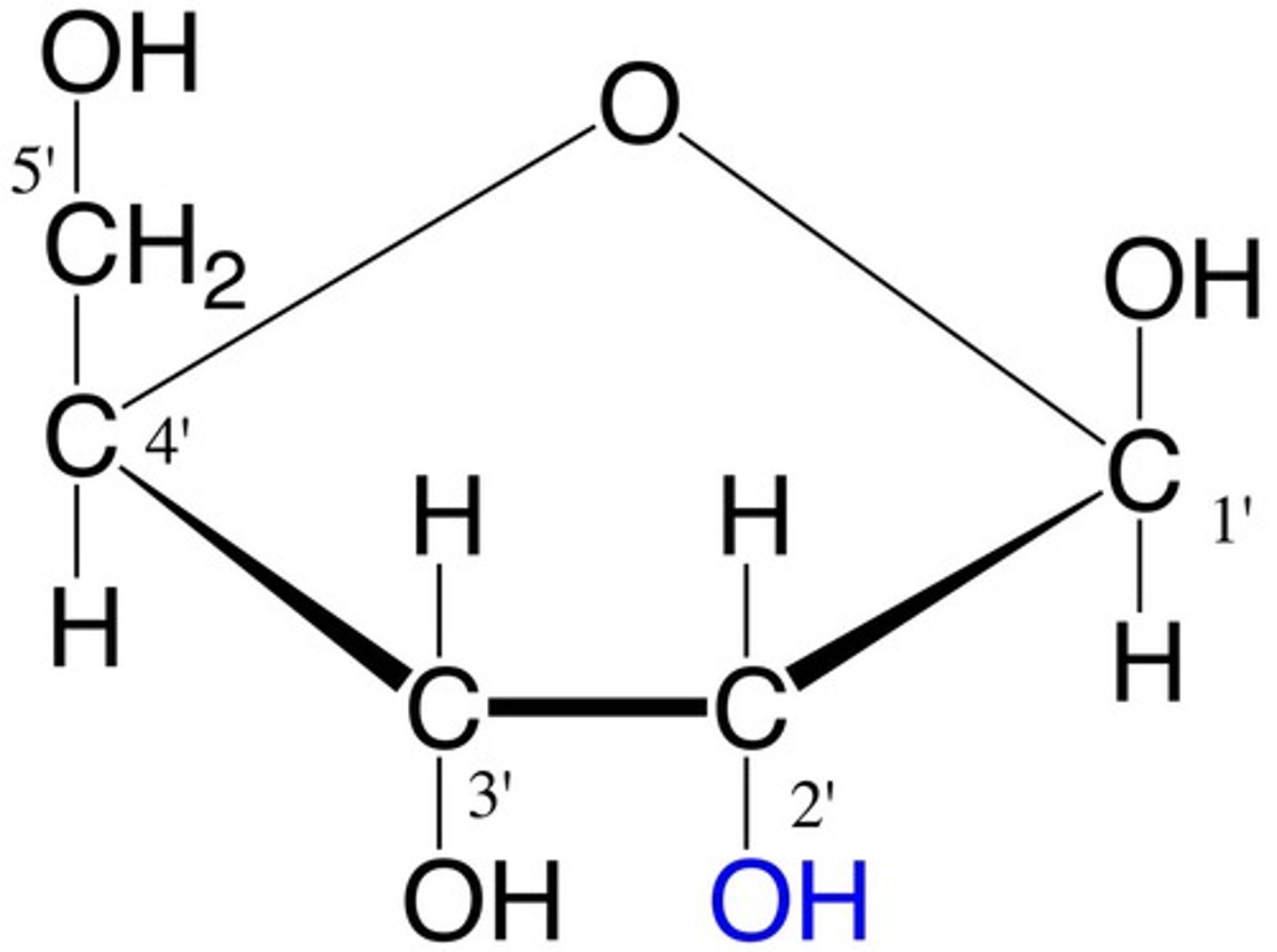
Monosaccharide cyclic structures
In aqueous solutions (in the body), aldotetroses and all monosaccharides (aldo or keto) with 5+ backbone carbon atoms cyclize
-A covalent bond forms between the carbonyl group and the oxygen of a hydroxyl group
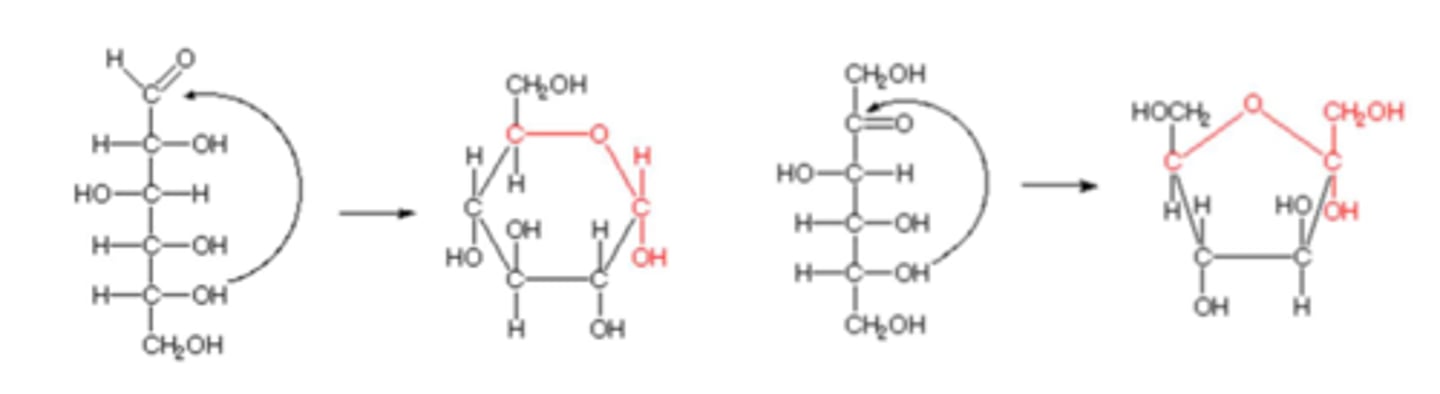
Anomeric carbon
The aldehyde or ketone carbon in the open chain form
-Upon cyclization, they are a chiral carbon adjacent to the ring oxygen and the hydroxyl group
Anomers
Isomers whose only difference is at the anomeric carbon
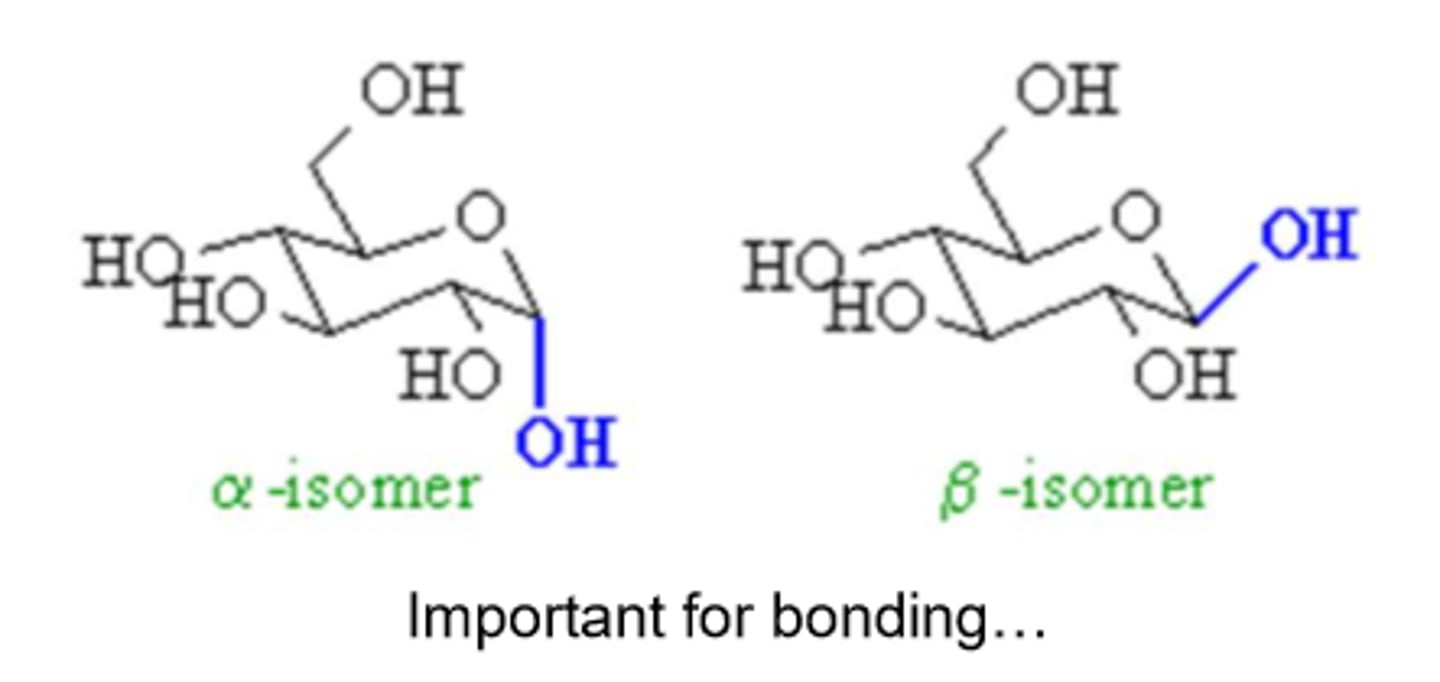
What are the two stereoisomeric configurations produced by the cyclization of monosaccharides?
α and β
How can you distinguish between α and β forms in monosaccharides?
By looking at the orientation of the -OH group at the anomeric center relative to the -CH2OH group
α-form
the -OH group at the anomeric center is on the opposite side of the -CH2OH group
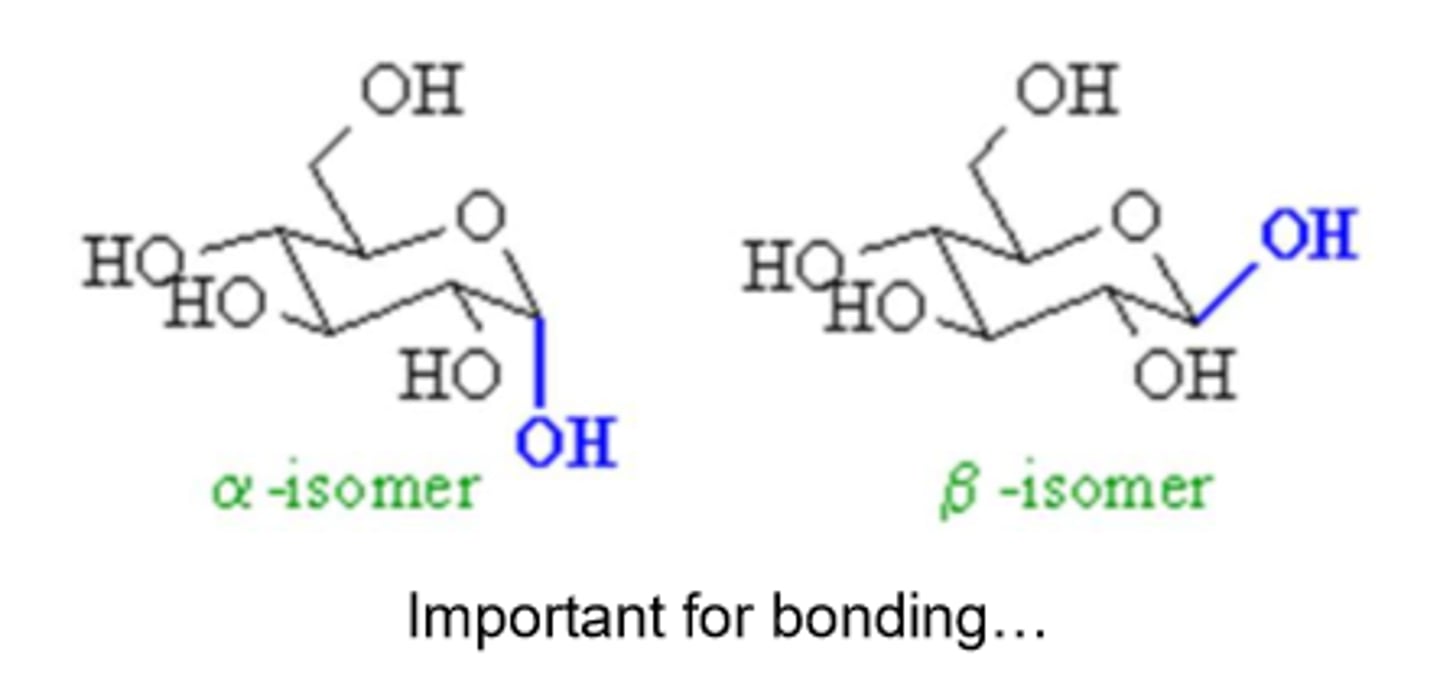
β-form
the -OH group at the anomeric center is on the same side of the -CH2OH group
Pyranoses
six-membered ring compounds
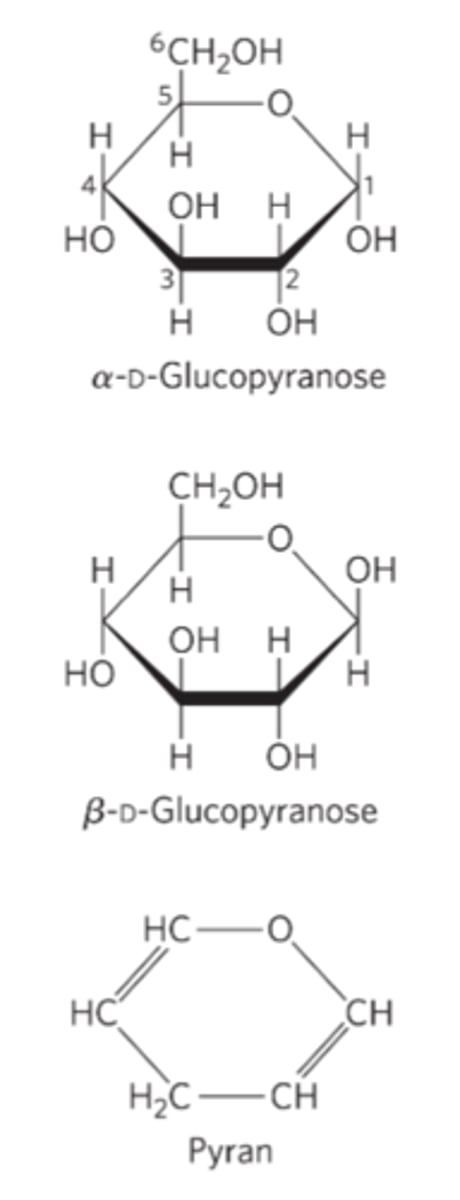
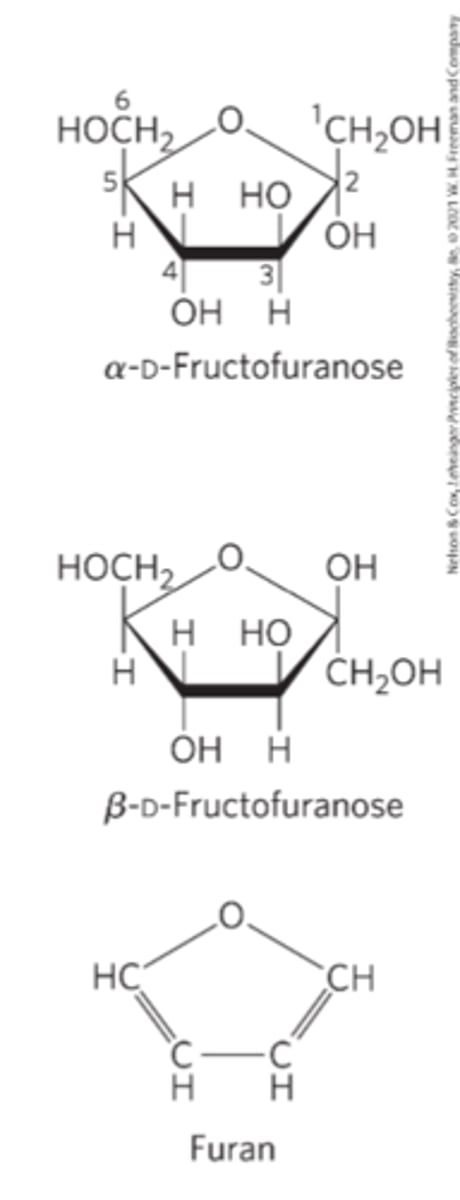
Furanoses
five-membered ring compounds
Haworth perspective formulas
a more accurate representation of cyclic sugar structure than Fischer projections
-Six-membered ring is tilted
-Bonds closest to the reader are drawn thicker than those father away
polysaccharides 3D structure
assume 3D structure with the lowest-energy conformation
-Determined by covalent bonds, hydrogen bonds, charge interactions, and steric factors
-Starch-> helical structure stabilized by internal hydrogen bonds
-Cellulose->extended structure with hydrogen bonds
Pyranose rings
tend to assume either of two "chair" conformations
-Interconvertible without breaking covalent bonds
-Transition requires energy input
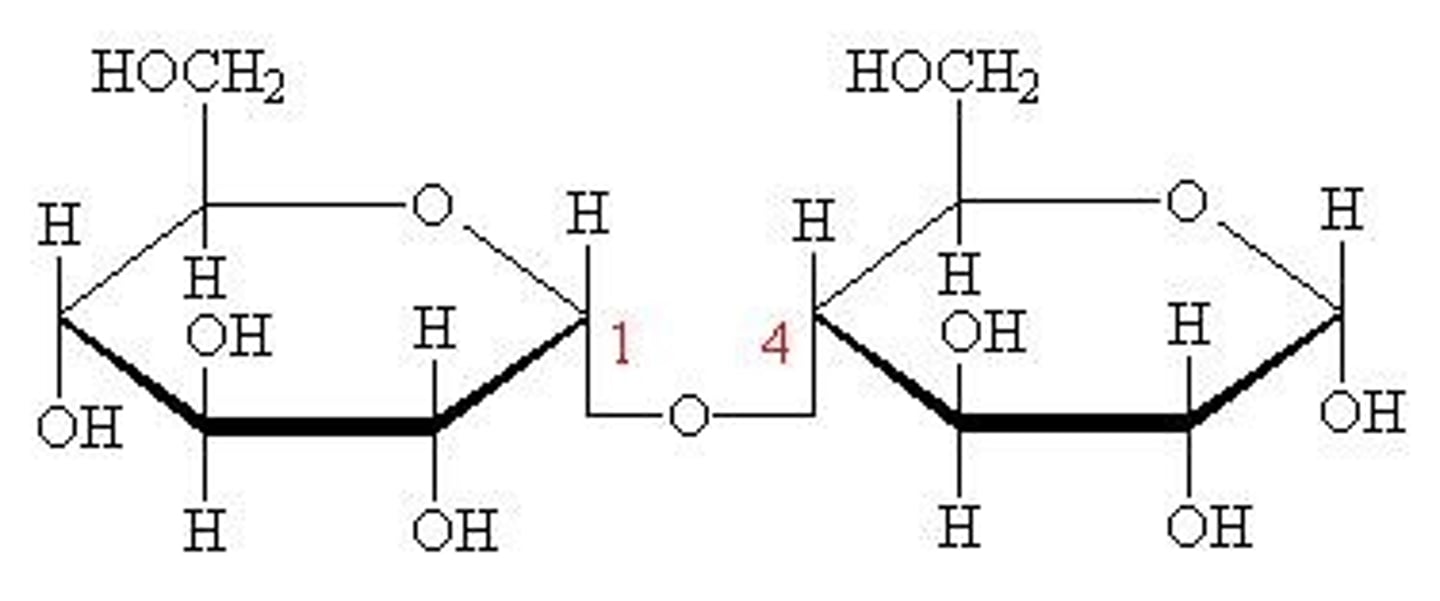
O-glycosidic bond
covalent linkage joining two monosaccharides
-The Hydroxyl group of one sugar molecule reacts with the anomeric carbon of the other
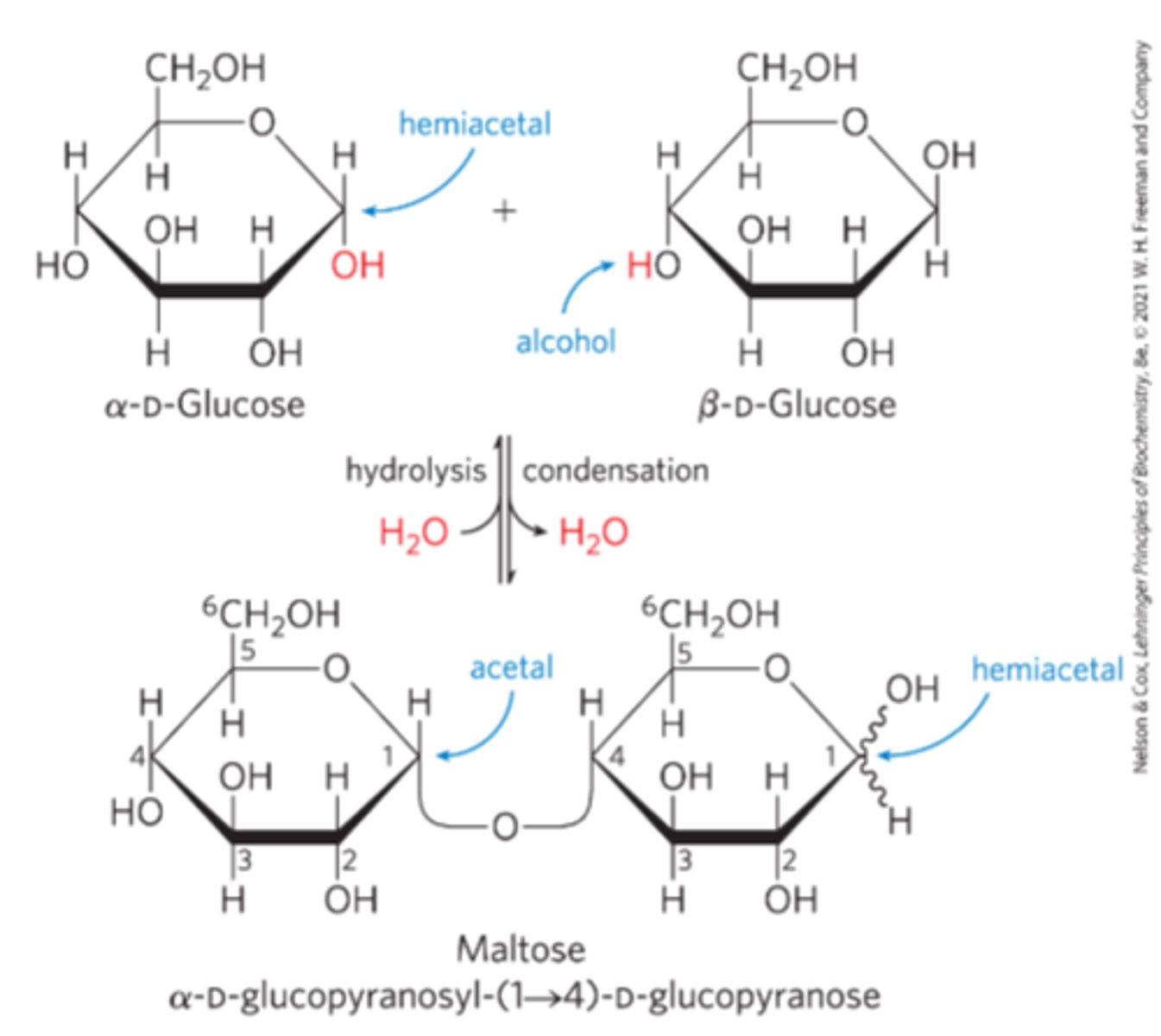
Glycosidic bonds
covalent ether (R-O-R') bonds joining a carbohydrate molecule to another group
-May or may not be another carbohydrate
-two forms: α and β
α glycosidic bonds
hydroxyl groups are opposite of CH2OH groups
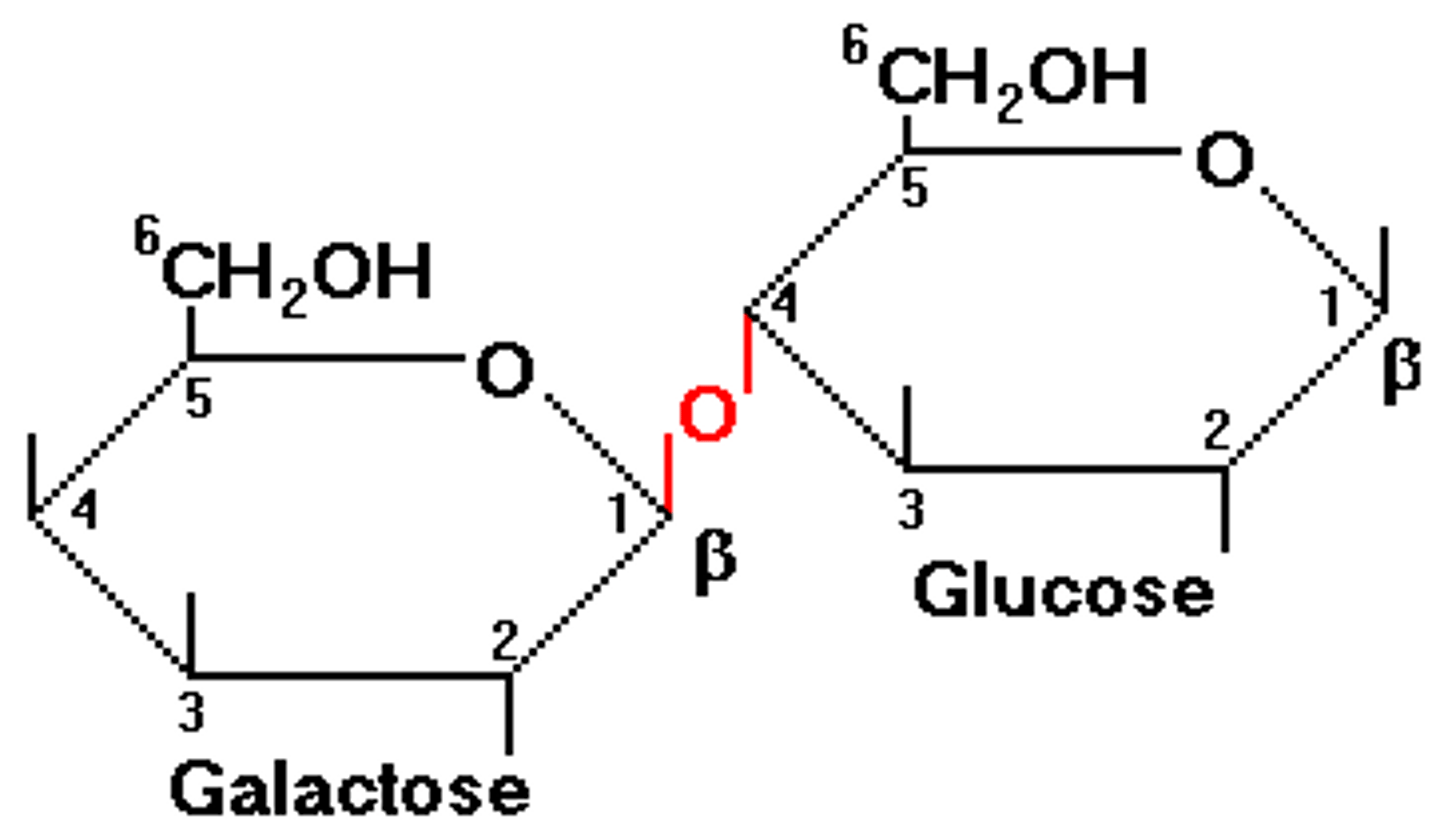
β glycosidic bonds
hydroxyl groups are on the same side of CH2OH groups
Monosaccharides
"simple sugars"
-Polyhydroxy aldehyde or polyhydroxy ketone unit
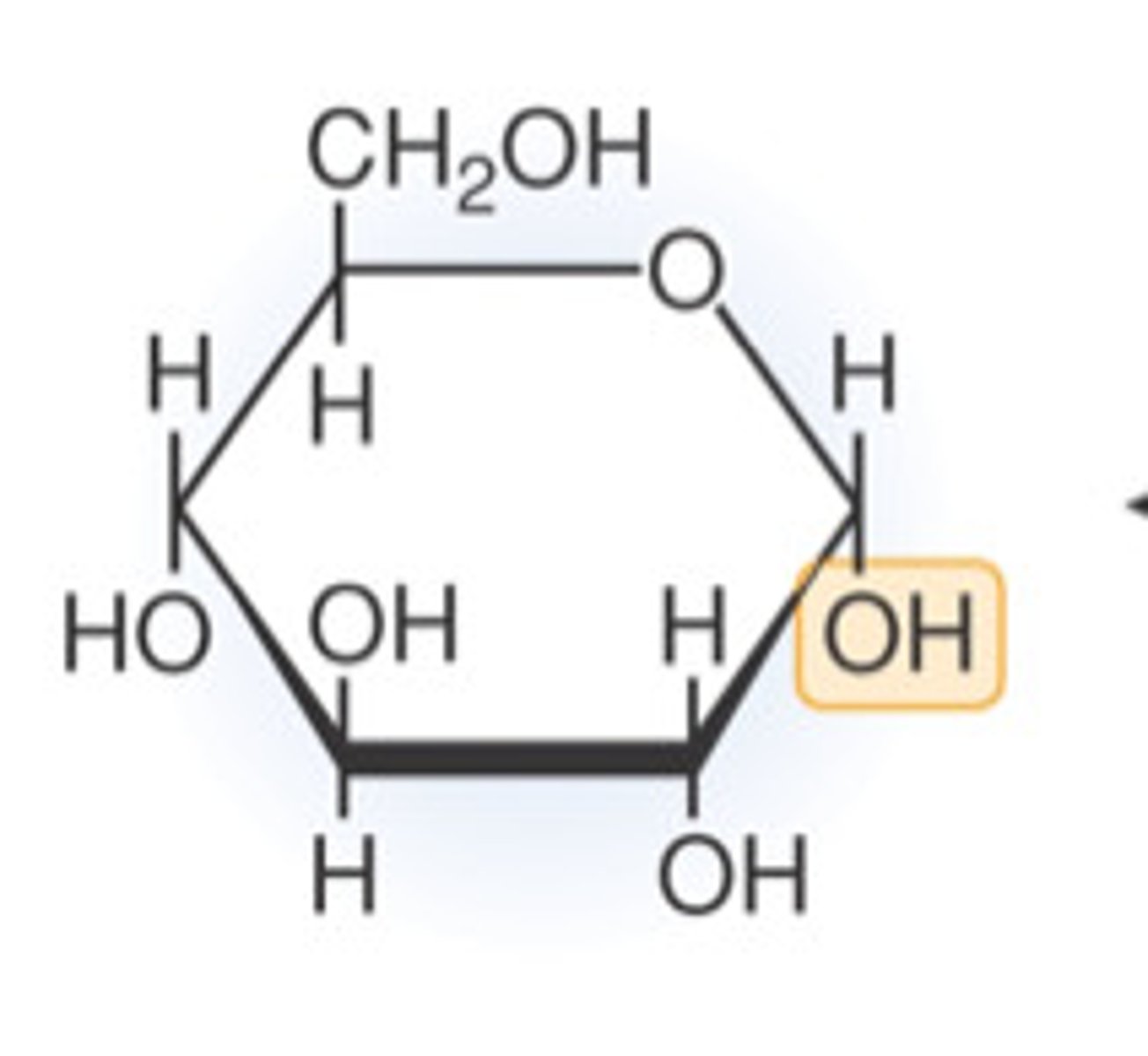
Glucose
monosaccharide
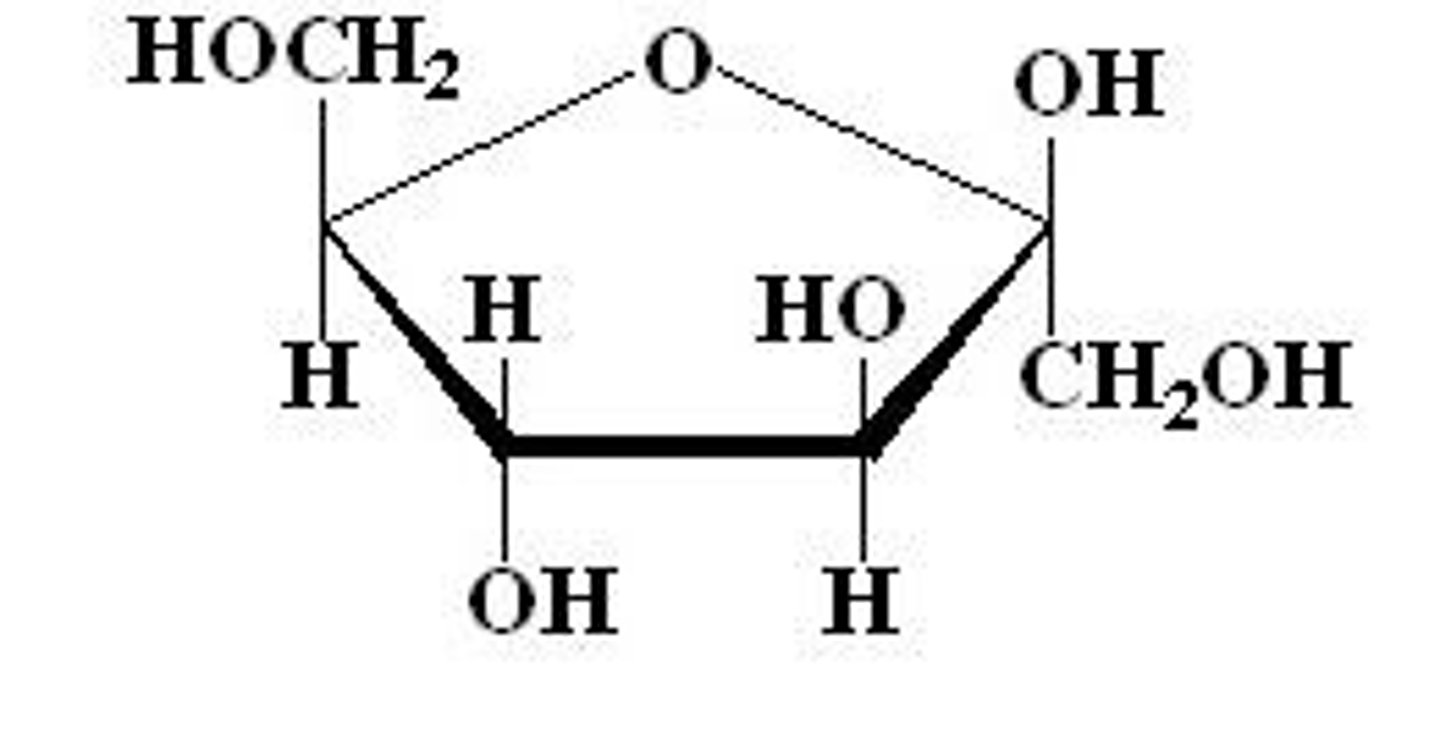
Fructose
monosaccharide
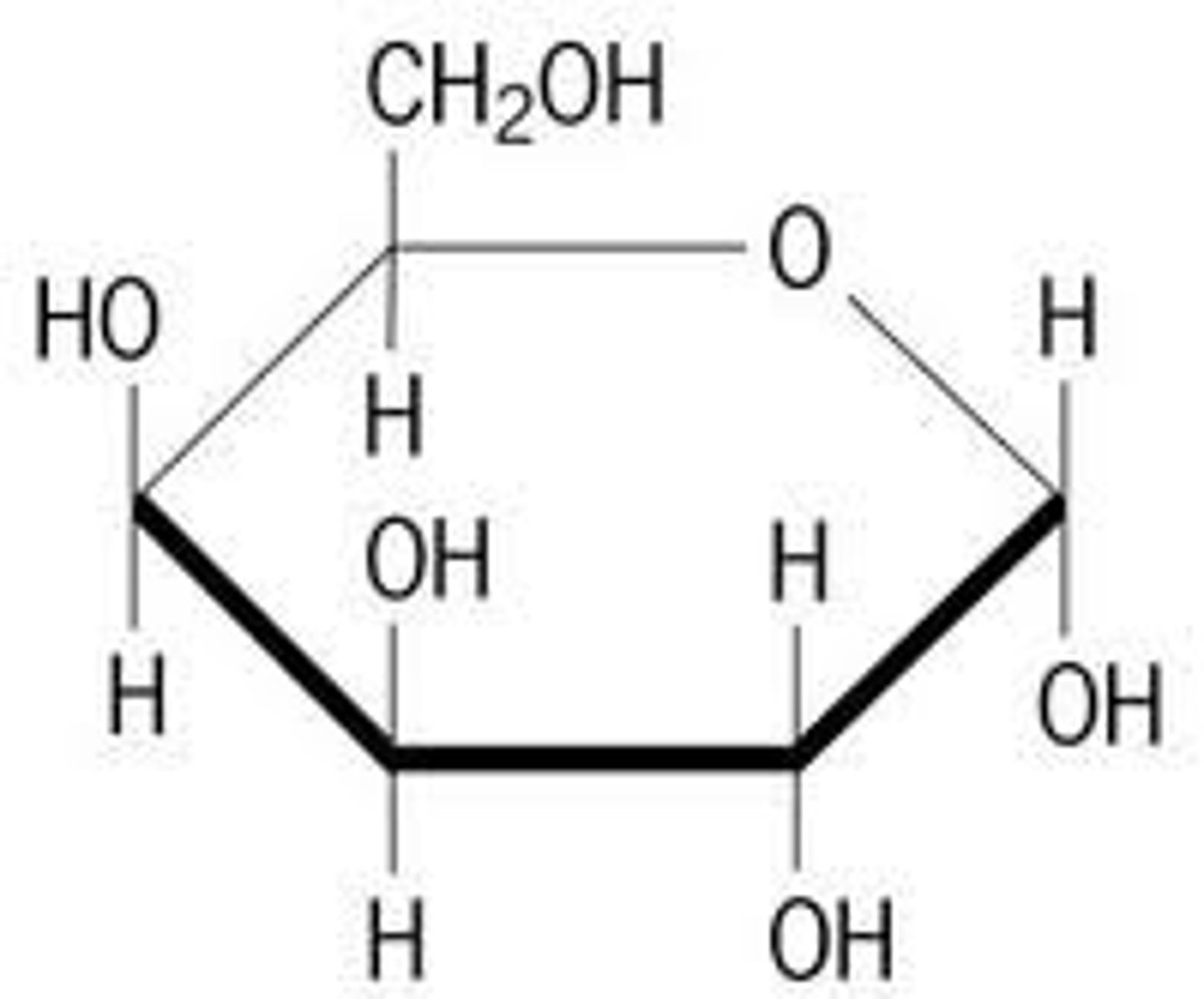
Galactose
monosaccharide
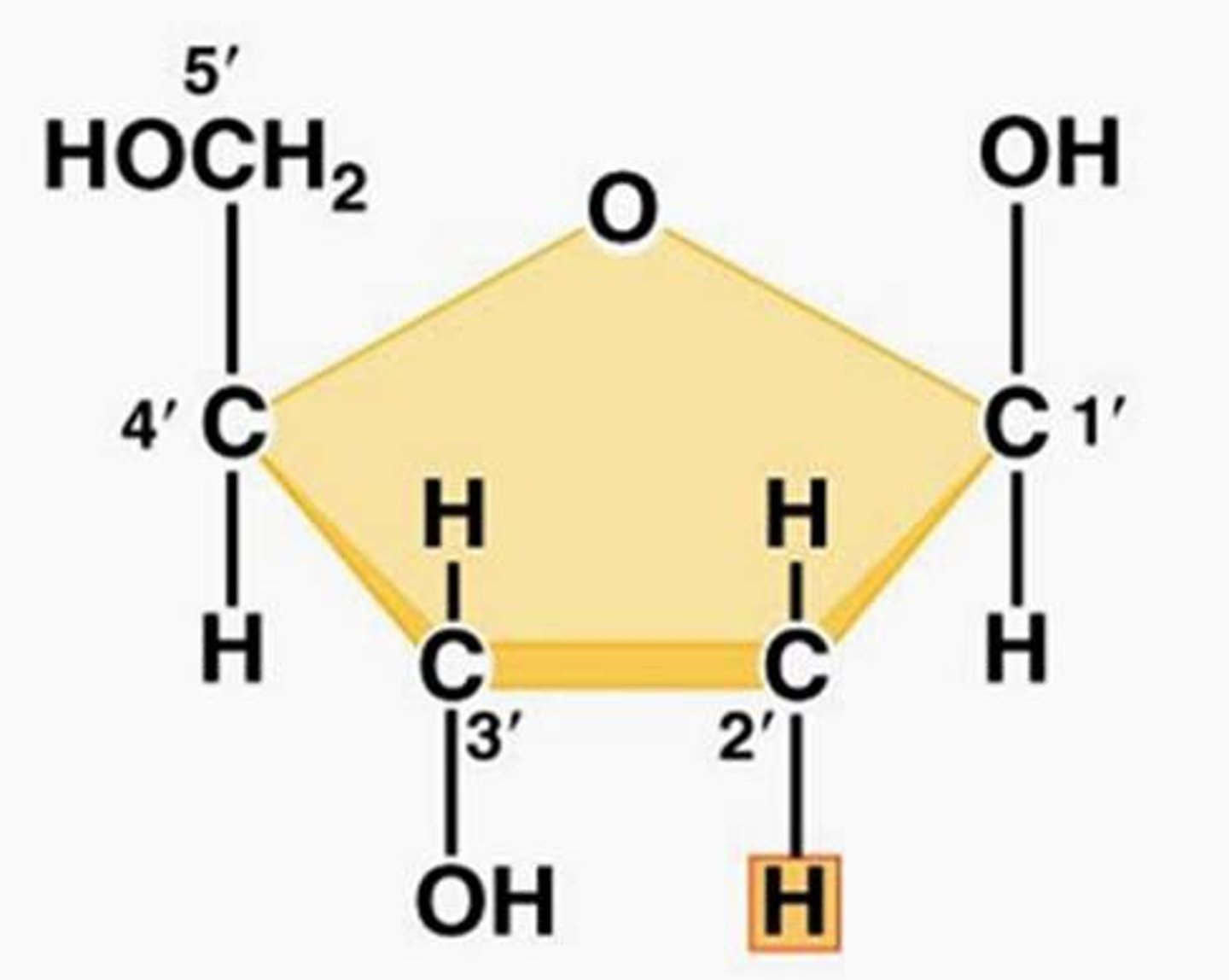
Deoxyribose
monosaccharide
ribose
monosaccharide
Oligosaccharides
Short chains of monosaccharide units/residues joined by glycosidic bonds (a bond that binds a sugar to another molecule)
Disaccharides
oligosaccharides with two monosaccharide units
maltose
disaccharide
glucose and glucose
lactose
disaccharide
galactose and glucose
sucrose
disaccharide
glucose and fructose
Reducing end
the end of a disaccharide or polysaccharide chain with a free (unattached) anomeric carbon
Reducing surgars
-are reducing agents
-they react with other molecules and donate electrons
-contain hemiacetal groups
-Used in some blood sugar testing
e.g., Lactose
Nonreducing sugar
e.g., Sucrose
-No free hemiacetal
-Can still form glycosidic bonds, just not donate electrons
Polysaccharides
sugar polymers with 10+ monosaccharide units
-ex: cellulose (linear), glycogen (branched)
-Most carbohydrates in nature
-Also known as "glycans"
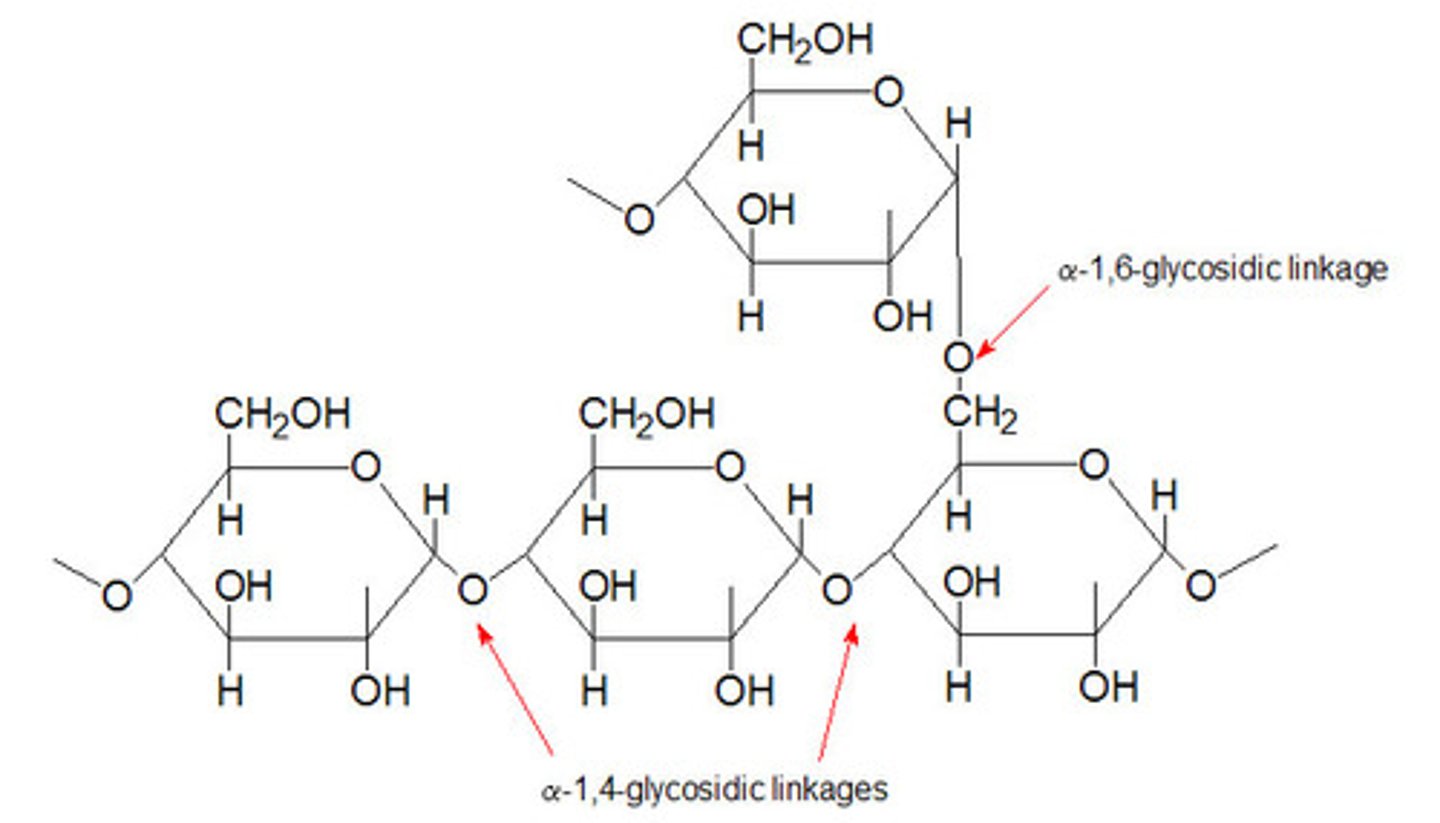
Starch
Made of two glucose polymers, amylose and amylopectin.
10%-30% amylose (D-glucose units joined by the α-1,4-glycosidic linkages) and 70%-90% amylopectin (D-glucose with α-1,4-glycosidic bonds but with occasional α-1,6-glycosidic bonds)
-Over 50% of our dietary carbs
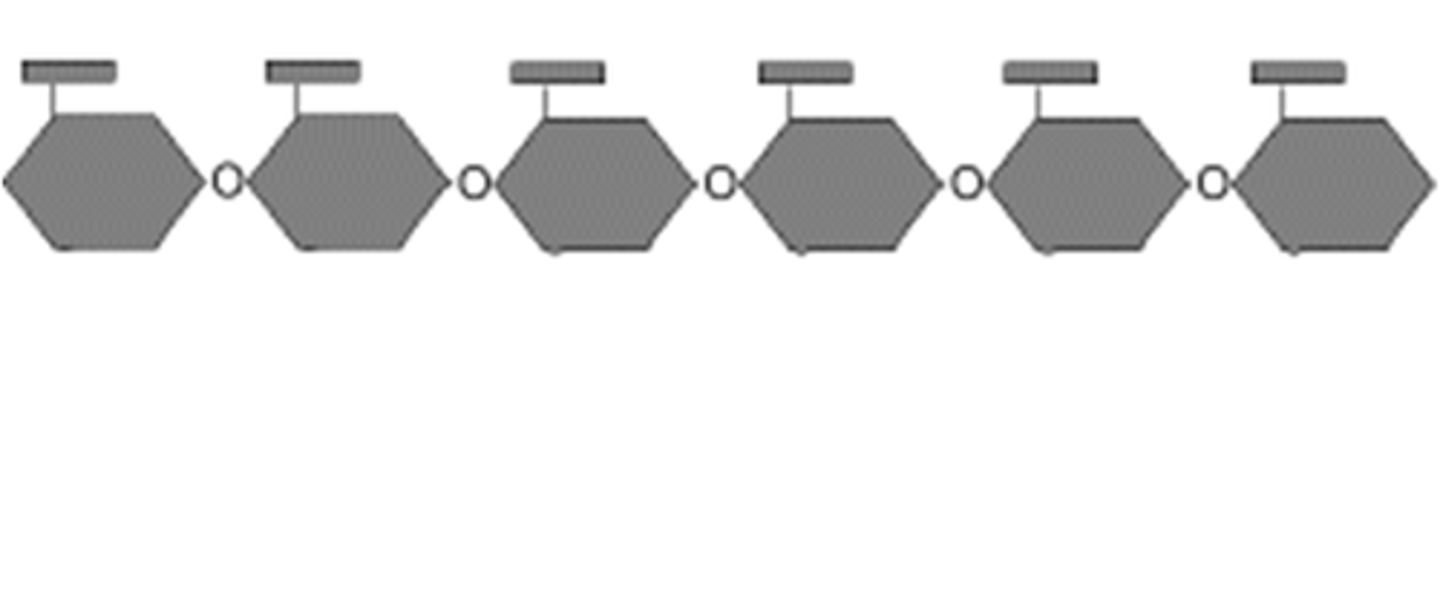
Polysaccharide conformations
assume the lowest energy conformations
-Structures are determined by covalent bonds, hydrogen bonds, charge interactions, and steric factors
-Starch folds into a helical structure stabilized by hydrogen bonds
-Hydrogen bonds are important in forming cellulose
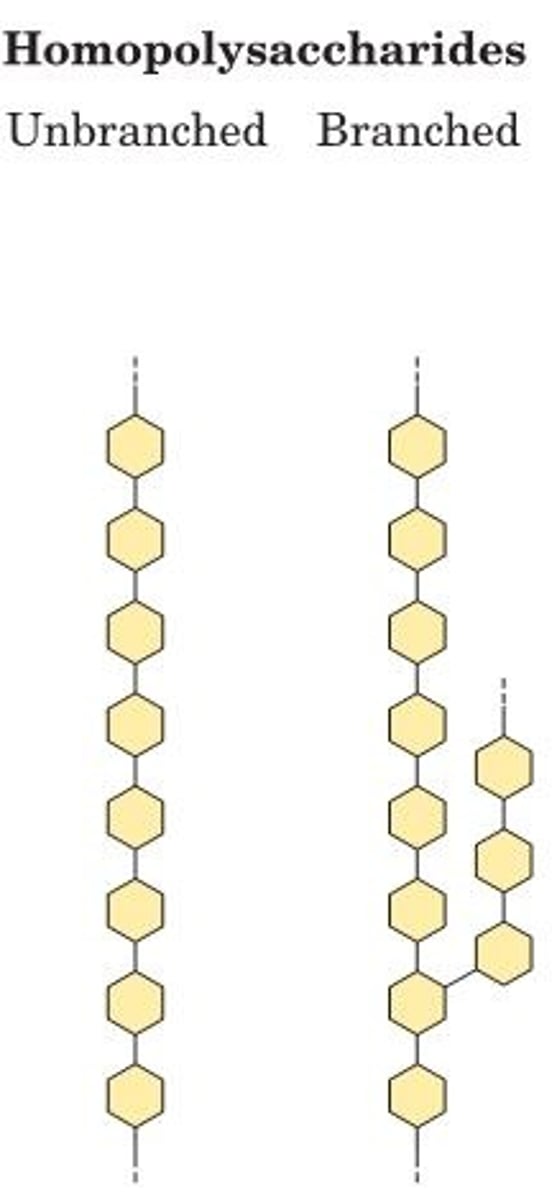
Homopolysaccharides
contain only a single monomeric sugar species
-Serve as storage forms and structural elements
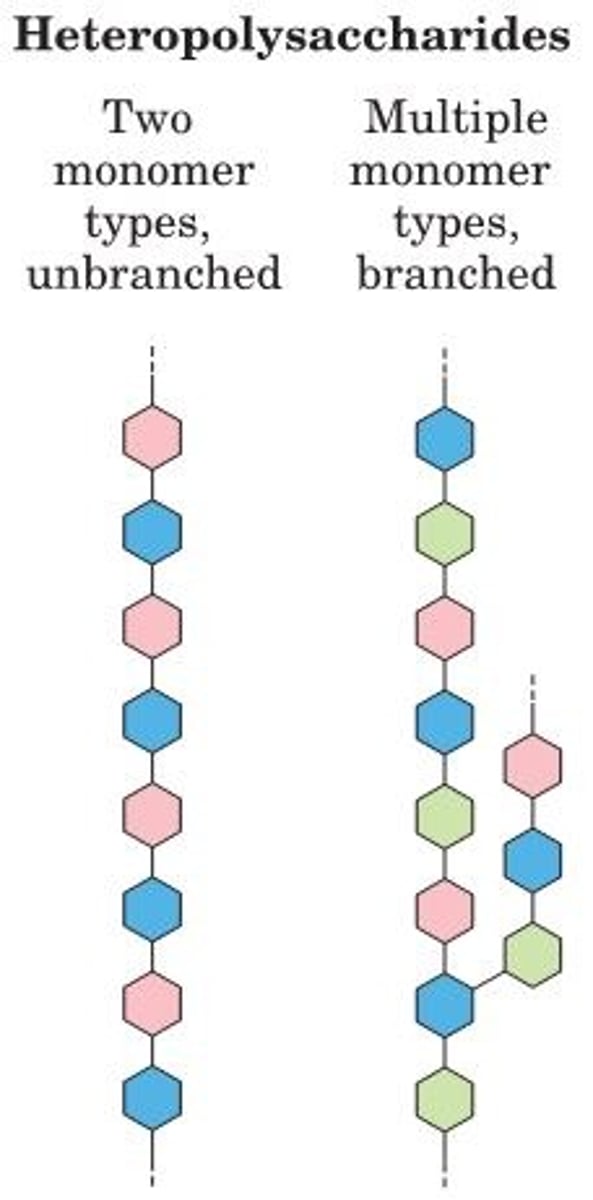
Heteropolysaccharides
contain 2+ kinds of monomers
-Provide structural support
Building polysaccharides
Structure is determined by their biosynthetic enzymes
-There is no template!
-In contrast with DNA, RNA, and proteins, which are synthesized using templates that direct their sequence.
Storing sugars
Storage of polymeric sugars prevents the high osmolarity that occurs when storing sugar monomers
-If the glucose in liver glycogen were monomeric, the glycose concentration in liver would be so high that cells would swell and lyse from the entry of water by osmosis.
storage polysaccharides
starch (plant cells) and glycogen (animal cells)
-Both are heavily hydrated- exposed hydroxyl groups hydrogen bond with water.
Amylose
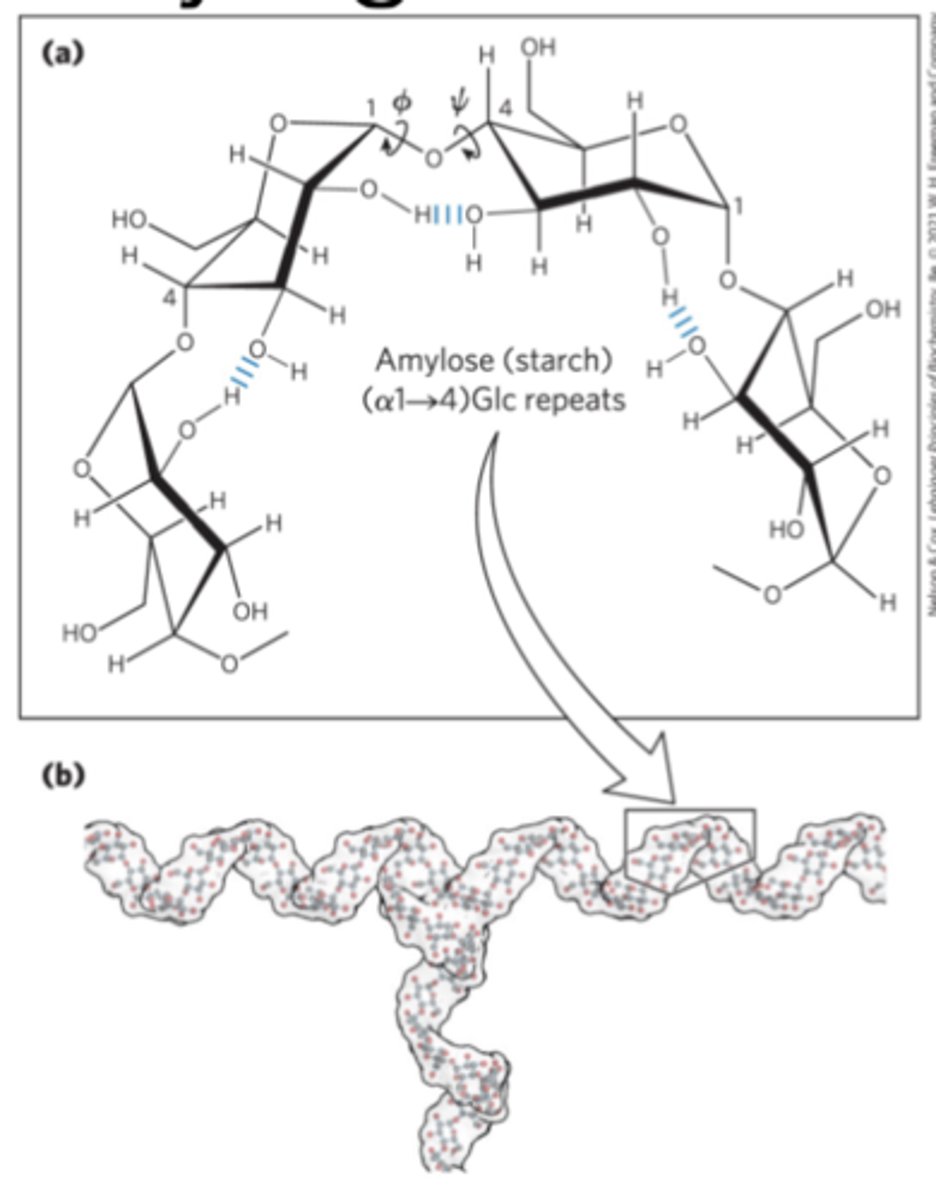
long, unbranched chains of D-glucose residues connected by (α1->4) linkages
Amylopectin
larger than amylose with (α1->4) linkages between glucose residues and highly branched due to (α1->6) linkages.
-In plants
Glycogen
polymer of (α1->4)-linked glucose subunits, with (α1->6)-linked branches
-More extensively branched
-More compact than starch (needs to fit in your liver)
-In animals
Helical structure of starch and glycogen
most stable three-dimensional structure for the (α1->4)-linked chains of starch and glycogen
-Six residues/turn
Cellulose
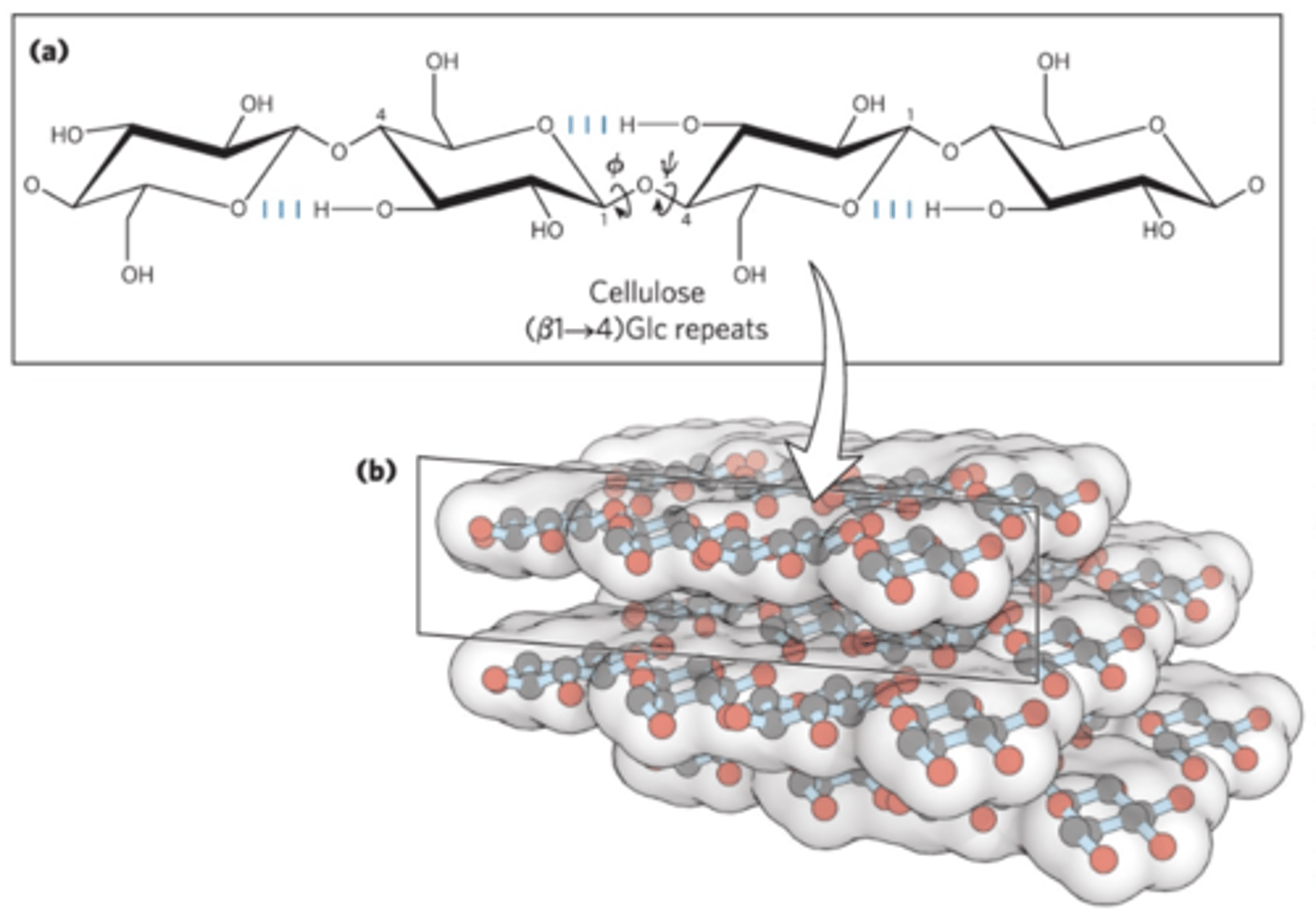
a tough, fibrous, water-insoluble glucose polysaccharide
-Long, linear, unbranched homopolysaccharide
-Glucose residues have the β configuration
-Linked by (β1->4) glycosidic bonds
-Animals do not have the enzyme to hydrolyze (β1->4) glycosidic bonds
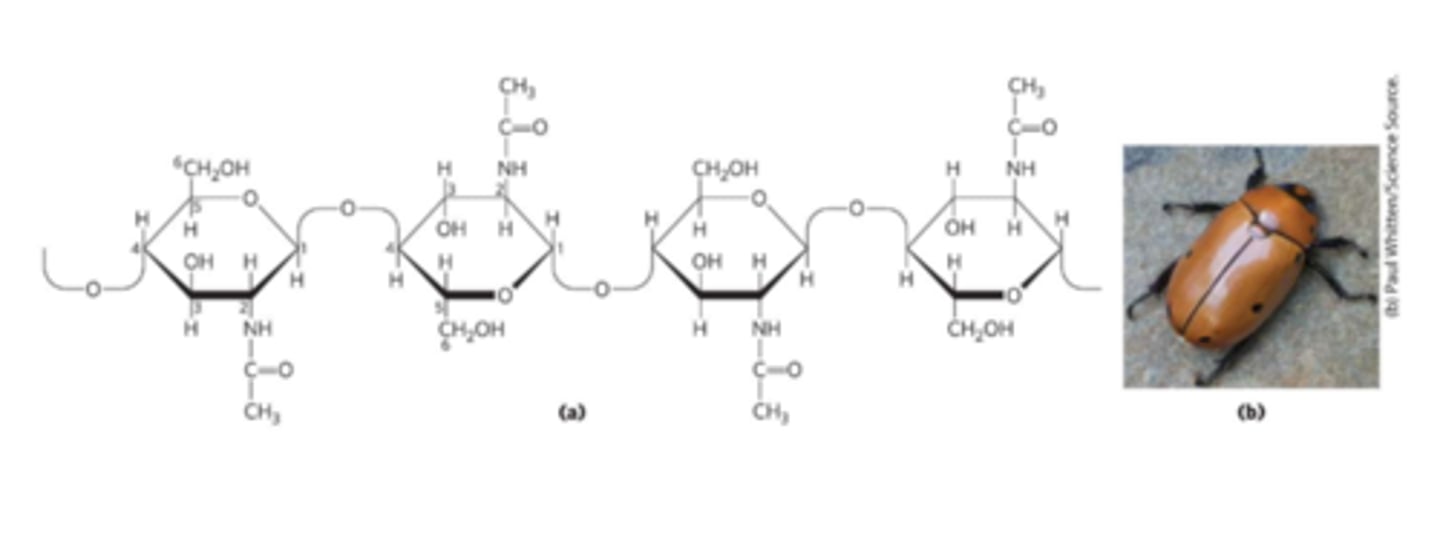
Chitin
Linear homopolysaccharide compose of N-acetylglucosamine residues in (β1->4) linkage
-Acetylated amino group makes chitin more hydrophobic and water-resistant than cellulose
-Exoskeletons
Linear structure of cellulose
Most stable conformation is a straight, extended chain
-Each chair is turned 180 relative to its neighbors
-Stabilized by hydrogen bonds
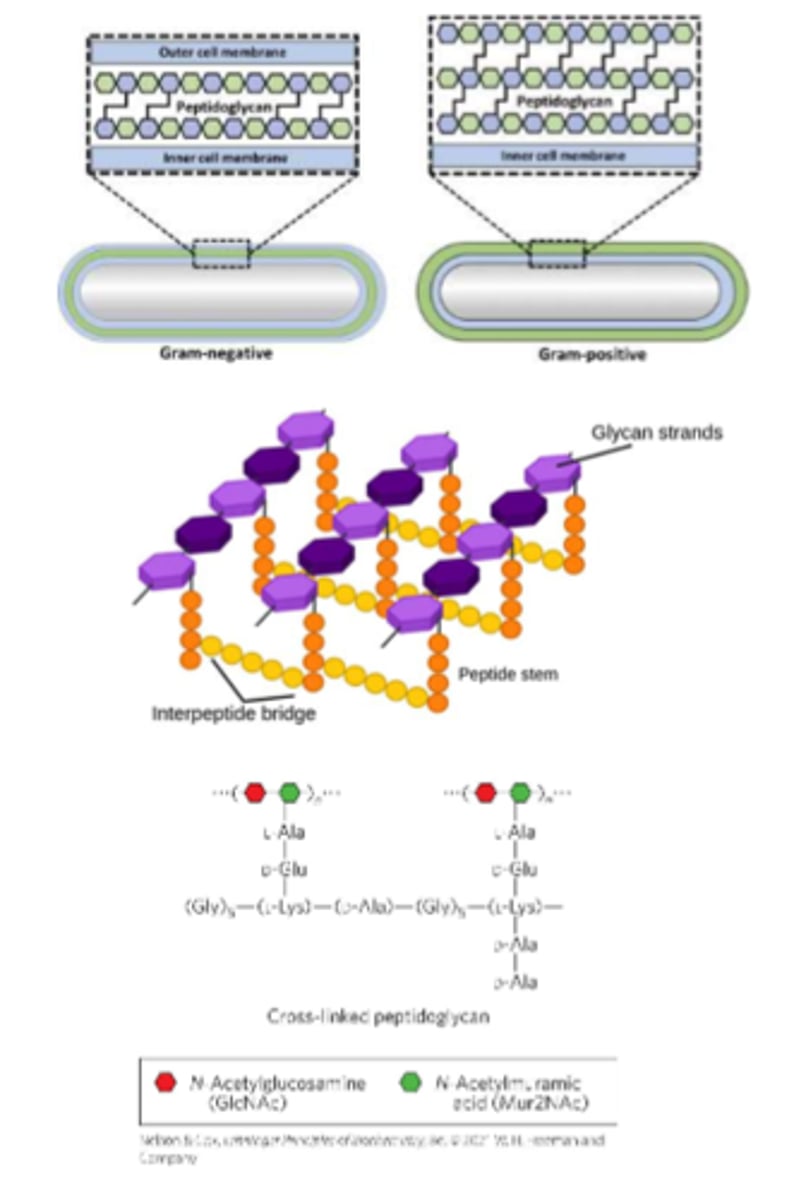
Peptidoglycan
is a rigid component of bacterial cell walls
-Heteropolymer
-Alternating (β1->4)-linked N-acetylglucosamine and N-acetylmuramic acid residues
-Cross-linked by short peptides - provides structural stability
Extracellular matrix (ECM)
A gel-like material in the extracellular space of tissues that holds cells together and provides a porous pathway for nutrient and O2 diffusion
-Composed of an interlocking meshwork of heteropolysaccharides and fibrous proteins
Basement membrane
specialized ECM that supports epithelial cells also contains heteropolysaccharides
Glycosaminoglycans (GAGs)
Heteropolysaccharides in ECM
-Linear polymers composed of repeating disaccharide units
-Highly polar and thus attract water
-Used as lubricants or shock absorbers in the body
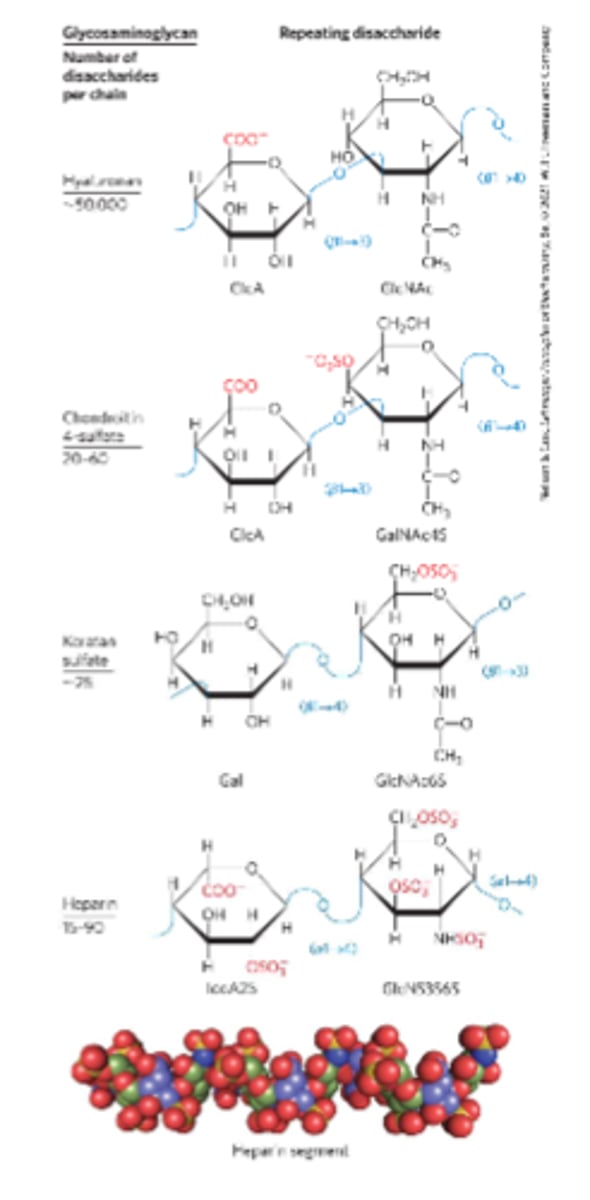
Hyaluronan (hyaluronic acid)
Alternating residues of D-glucuronic acid and N-acetylglucosamine
-Used to treat burns and promote wound healing
-Type of GAG
Heparan sulfate
A highly sulfated glycosaminoglycan
-Sulfated residues allow the molecule to interact specifically with proteins
-Used as a therapeutic agent to inhibit coagulation of blood
-Provide viscosity, adhesiveness, and tensile strength to the ECM
Proteoglycan Aggregates
Supramolecular assemblies of many proteins bound tr a single molecule of hyaluronan
-Aggrecan interacts strongly with collagen on the ECM of cartilage
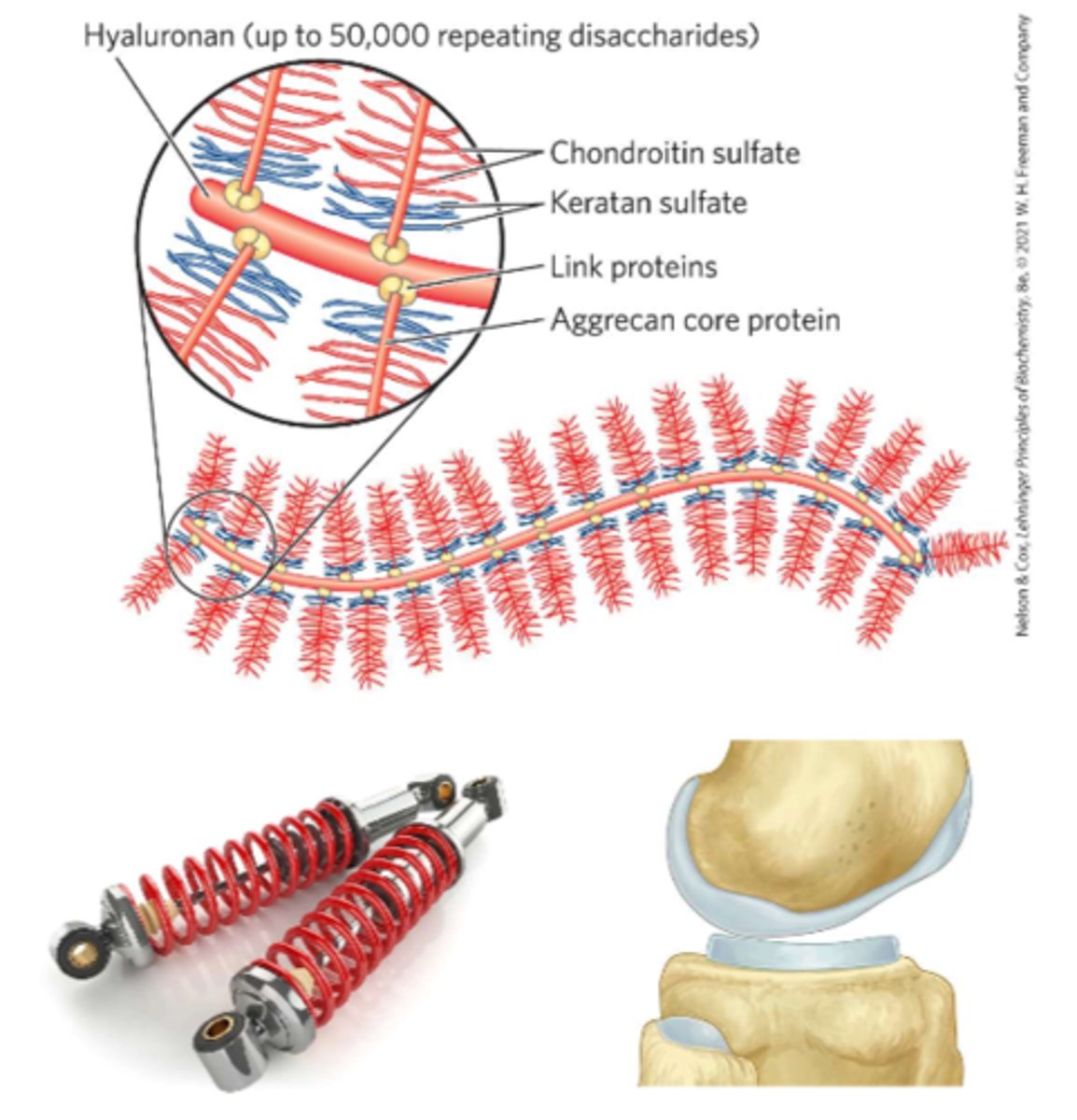
Antithrombin
inhibits thrombin (a protease)
-Only in the presence of heparan sulfate
-Heparan sulfate enhancement of binding of thrombin to antithrombin
Dextran
structural: in bacteria, gives rigidity and strength to cell envelope
Glycoconjugate
are biologically active molecules consisting of an informational carbohydrate joined to a protein or lipid
Proteoglycans
Macromolecules of the cell surface of ECM
- less than or equal to 1 sulfated GAG covalently bonded to a protein
-A major component of all extracellular matrices
Glycoproteins
Oligosaccharides covalently bonded to a protein.
-Used for cell signaling
-Heterogenous
-Rich in information
Glycolipids
lipids with oligosaccharide (hydrophilic) head groups
-Often in the plasma membrane
Glycosphingolipids
A class of glycolipids with specific backbone structure
-Found in neurons
-Play a role in signal transduction
Interactions between cells and the ECM
- anchor cells to the ECM, providing the strength and elasticity of skin and joints
- provide paths that direct the migration of cells in developing tissue
- convey information in both directions across the plasma membrane
Glycoproteins in the body
-Antibodies
-Follicle-stimulating hormone (FSH)
-Luteinizing hormone (LH)
-Thyroid-stimulating hormone (TSH)
-Milk proteins E.g. major whey protein α-lactalbumin
-Mucins - O-linked oligosaccharide chains, Secreted or membrane bound, Present in most secretions
Glycobiology
the study of the structure and function of glycoconjugates
-The goal is to understand how cells use specific oligosaccharides to encode information about:
-Intracellular targeting of proteins
-Cell-cell interactions
-Cell differentiation and tissue development
-Extracellular signals
Glycomics
the systemic characterization of all carbohydrate components of a given cell or tissue, including those attached to proteins and to lipids
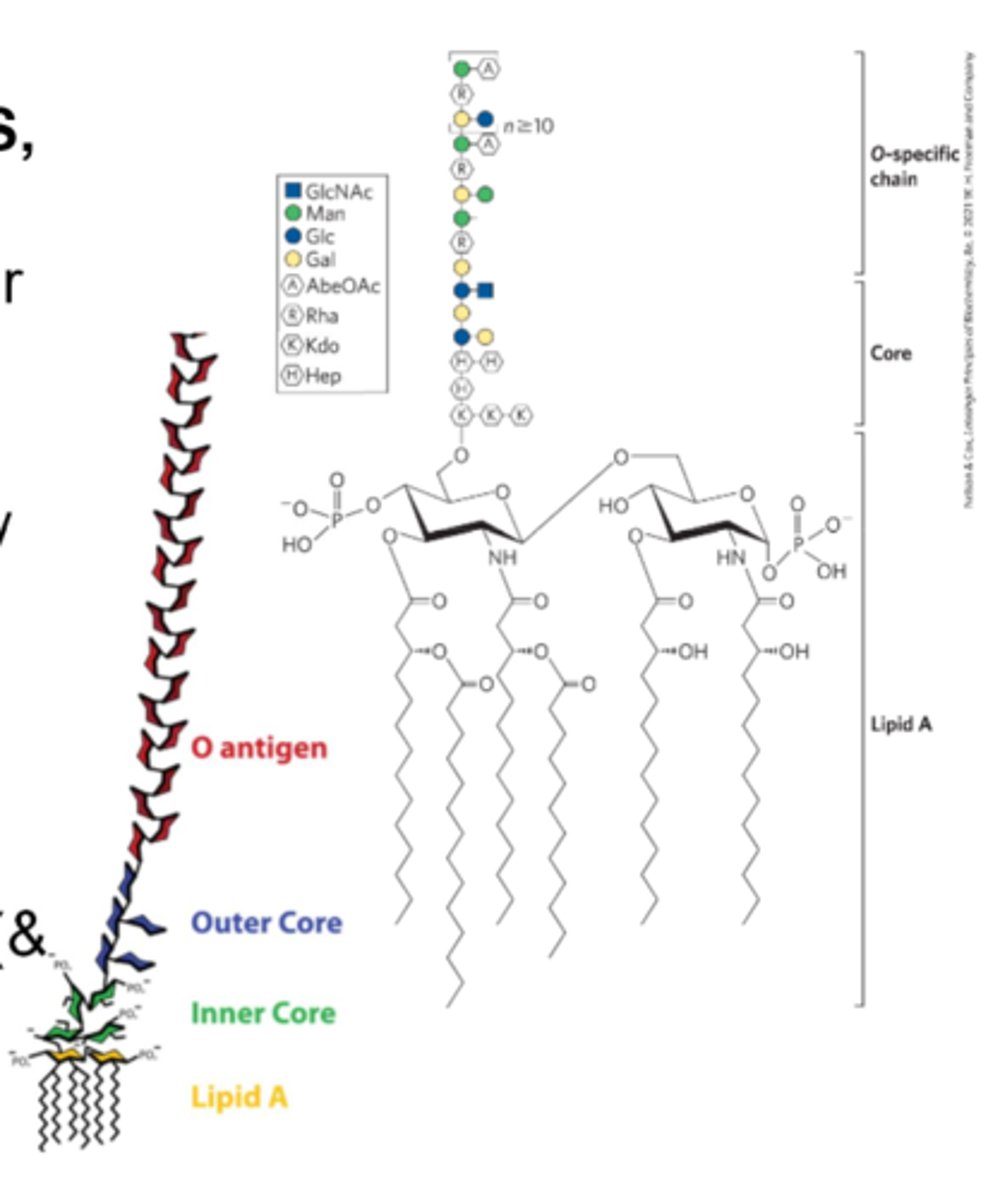
Lipopolysaccharide
(LPS, endotoxin): A dominant surface feature of the outer membrane of gram-negative bacteria
-O antigen is recognized by the immune system and antibodies are generated against it
-Potent
-Can cause septic shock (& other negative effects)
Oligosaccharides structures
Commonly branched, unlike nucleic acids or proteins
-Meaning no branching structural backbone
-Does not include not R groups, prosthetic groups, etc
variety of oligosaccharides
large variety due to:
-Stereochemistry and position of glycosidic bonds
-Type and orientation of substituent groups
-Number and type of branches
Lectins
sugar binding proteins
-bind specific sugars/polymers
-can read sugar sequence code and mediate biological processes -Bind carbohydrates with high specificity and with moderate to high affinity
Lectin functons
-Cell-cell recognition
-Signaling
-Adhesion
-Intracellular targeting of newly synthesized proteins
Selectins
a family of plasma membrane lectins
-Mediate cell-cell recognition and adhesion
-Move immune cells through the capillary wall
-Mediate inflammatory responses
-Mediate the rejection of transplanted organs - "Selectins are selective about organ transplantation"
Fibronectin
-A multifunctional adhesive glycoprotein ECM structure
-Involved in tissue repair, regulating cell motility, and embryogenesis
-Binds fibrin, herparan sulfate, collagen, and integrins
Integrins
transmembrane proteins that mediate signaling between cell interior and ECM molecules
Lectin-Ligand interactions
play a role in leukocyte movement
-Cells will express specific receptors when they need leukocytes
Lectin Multivalency
-Lectin-Carbohydrate interactions are highly specific and often multivalent
-Multivalent= Several possible binding sites/interactions
-A single lectin molecules has multiple carbohydrate binding domains - Increase total affinity
Blood Typing
determined by the presence or absence of specific carbohydrate antigens on the surface of red blood cells (RBCs)
-Lectins selectively bind to these carbohydrate structures
-This interaction helps identify the type of antigens present on the RBCs
-foreign blood will cause immune response if not compatible
Lectins in blood typing
A drop of blood is mixed with a lectin solution that binds to a specific blood antigen.
If the lectin binds to the antigens on the surface of red blood cells, agglutination occurs (indicates the presence of that specific antigen)
AB blood
universal recipient