chapter 1- covalent bonding and shapes of molecules
1/99
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
100 Terms
Aufbau Principle (quantum chemistry principle)
electrons occupy orbitals in order of increasing energy, starting with the lowest energy level first
Pauli Exclusion Principle (quantum chemistry principle)
no more than two electrons may be present in an orbital. If two electrons are present, their spins must be paired
Hund's Rule (quantum chemistry principle)
when orbitals are of equal energy are available but there are not enough electrons to fill up all of them, one electron fills each orbital before doubling up. The spins of the electrons in degenerate orbitals must be aligned
first ionization energy
The minimum amount of energy required to remove the first electron (most loosely held) from the outermost shell of a single neutral atom
valence shell
The outermost energy shell of an atom, containing the valence electrons involved in the chemical reactions of that atom (period # = # of shell, group #= # of valence electrons in valence shell) (middle elements have 2 valence electrons)
lewis dot structure
diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule
1. no. of valence electrons combined
2.Determine arrangement of atoms Carbon is often in the middle because is the least electronegative never hydrogen in the middle because it only can have one bond
Show the bonding electrons shared btw 2 atoms as single line btwn atoms
show lone pairs
IF needed make double or triple bods
make sure all atoms are bonded
Want a postive formal charge + need to subtract a negative valence electrons
Want a negative formal charge - need to add an electrons
difference between a coordinate covalent bond and a simple covalent bond
In a covalent bond, both atoms are contributing same number of electrons to the bond, but in a coordinate covalent bond, two electrons are donated by a single atom
Two electrons come from a single group or atom
Anion
Atom that gains electrons
Cation
Atom that loses electrons
Covalent Bonds
Electrons are shared btw Atoms
Non-Polar Covalent Bonds
An Atom Bons with Another Atom of the Same Element
Polar Covalent Bonds
Occurs when an atom bons with an atom of a different element
Electronegativity
The ability of an atom to attract electrons from another atom to form a chemical bond. (Fluorine)
Nonpolar covalent
less than 0.5
Polar Covalent
1.9-0.5
Ions Forms
Greater than 1.9
How to Find Electronegativity


Polar Covalent
The one is that is more electronegative is dipole negative because it can pull more electronegative

These molecules are polar
The direction of the dipole moment is to the moment is in the direction of the most electronegative atom
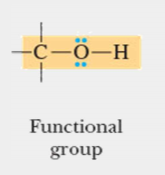
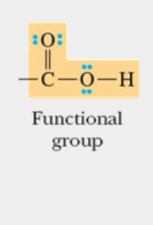
functional group
A specific configuration of atoms commonly attached to the carbon skeletons of organic molecules and involved in chemical reactions; basis of naming organic molecules; units by which compounds are divided into clases
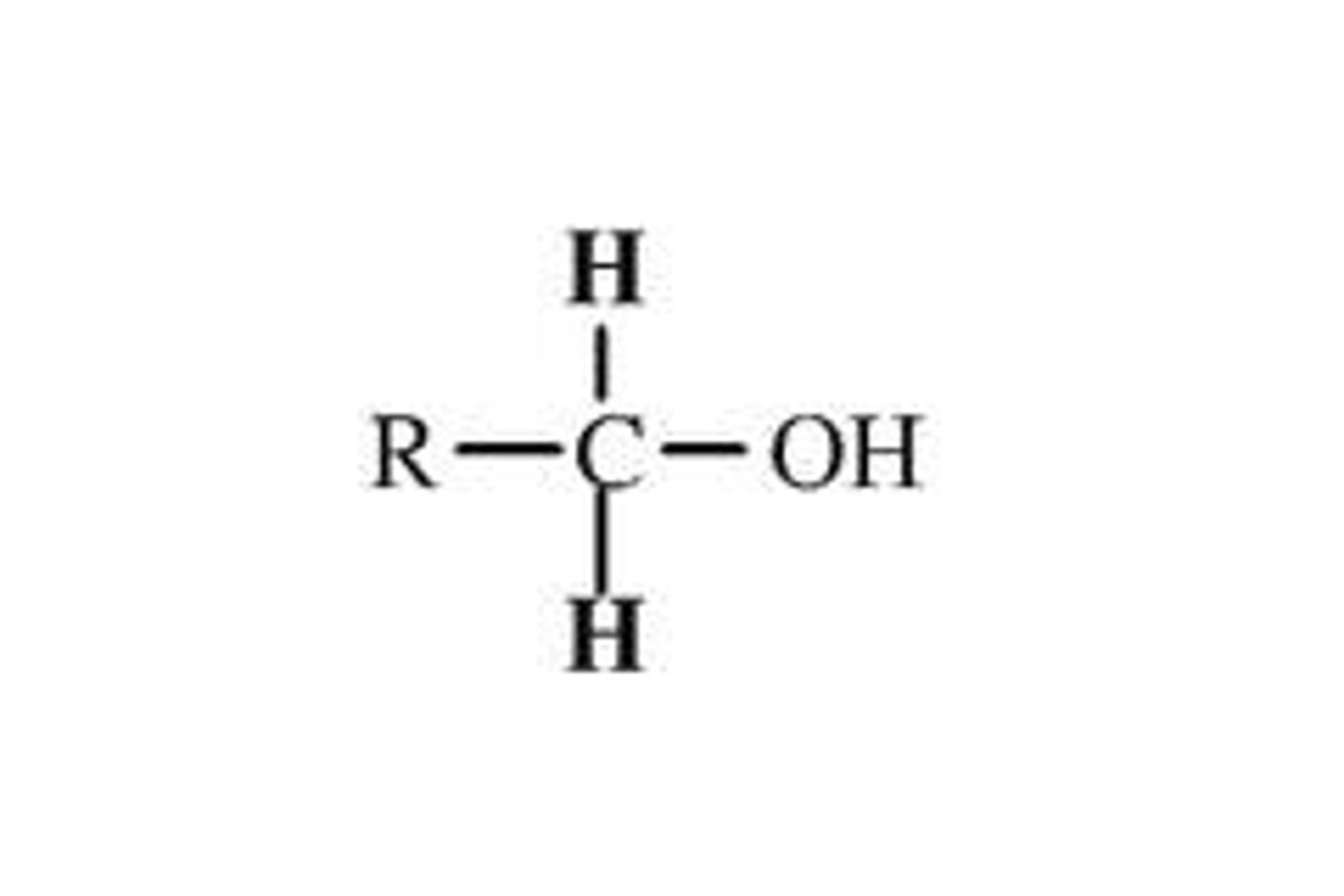
alcohol
R-OH, suffix- ol
primary alcohol
OH bonded to carbon that is bonded to one other carbon
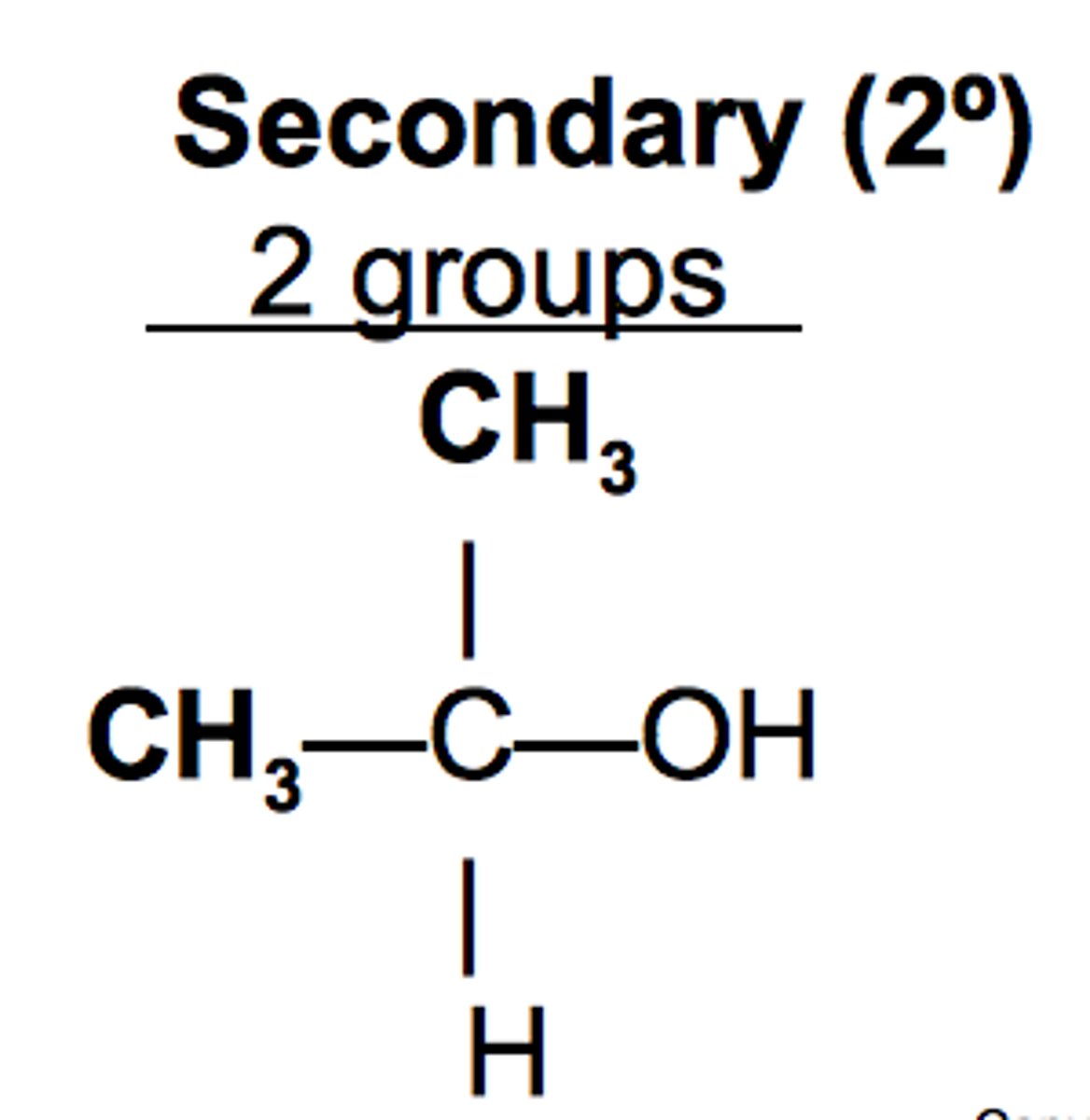
secondary alcohol
OH bonded to carbon that is bonded to two other carbons
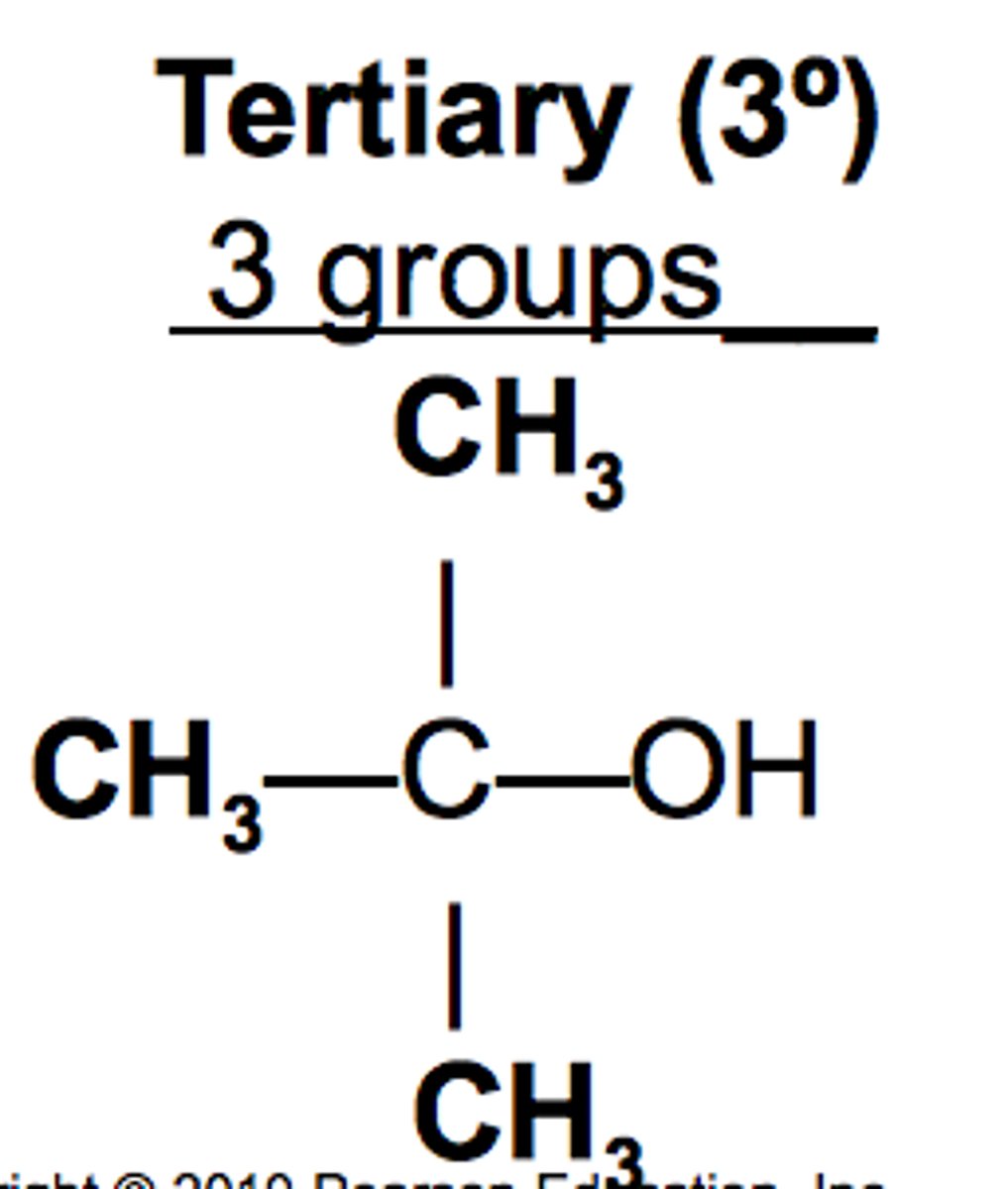
tertiary alcohol
OH bonded to carbon that is bonded to 3 other carbons
constitutional isomers
compounds with the same molecular formula but different connections among their atoms
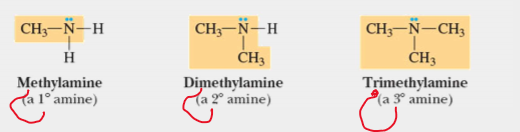
amines
contain amino group- nitrogen bonded to one, two, or three carbon atoms by a single bonds (ex: methylamine (primary), dimethyl amine (secondary) , trimethyl amine (tertiary)); RNH2
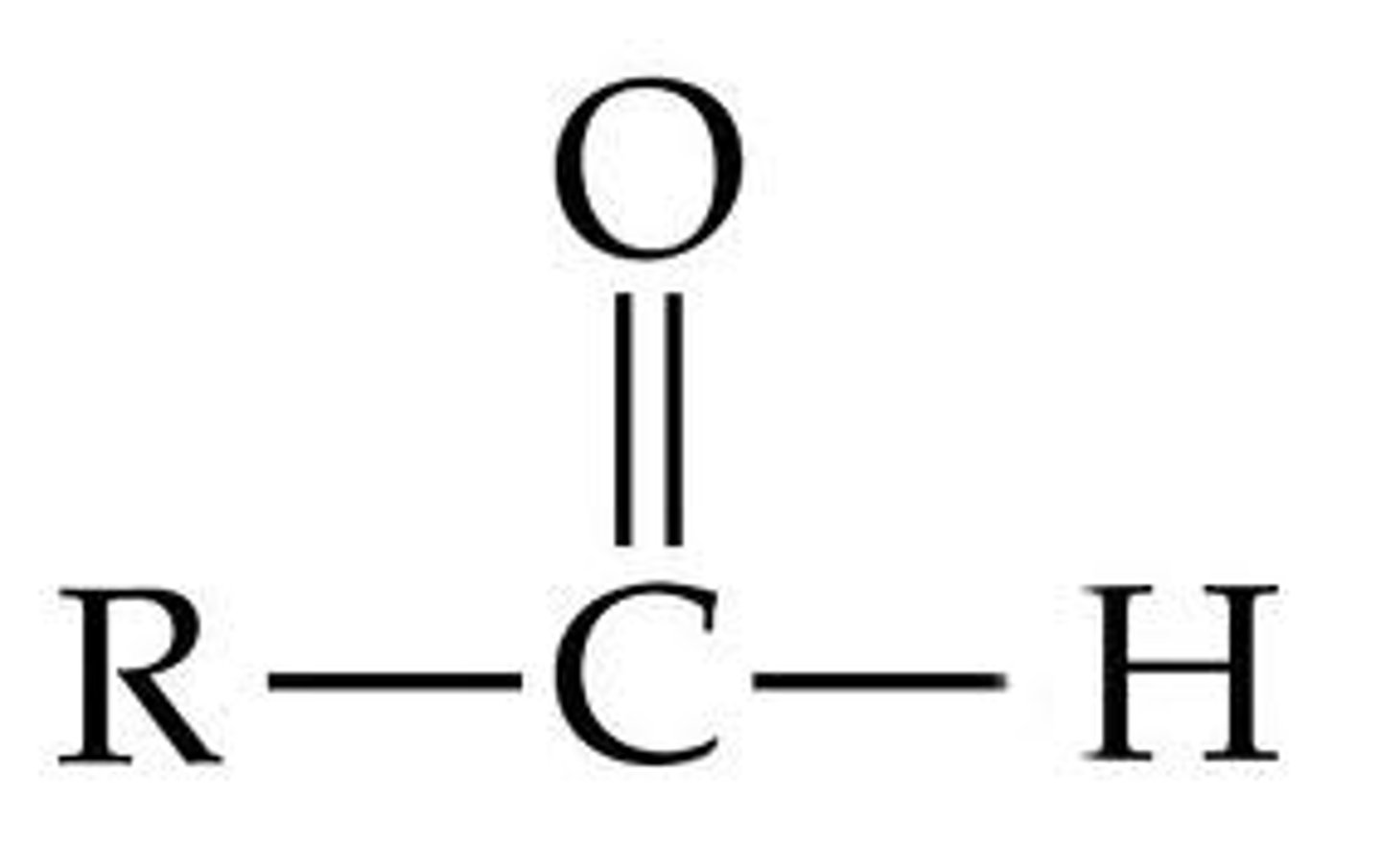
aldehyde
contains a carbonyl group (c double bond o) at the end of the molecule (ex: acetaldehyde); RCHO, suffix -al
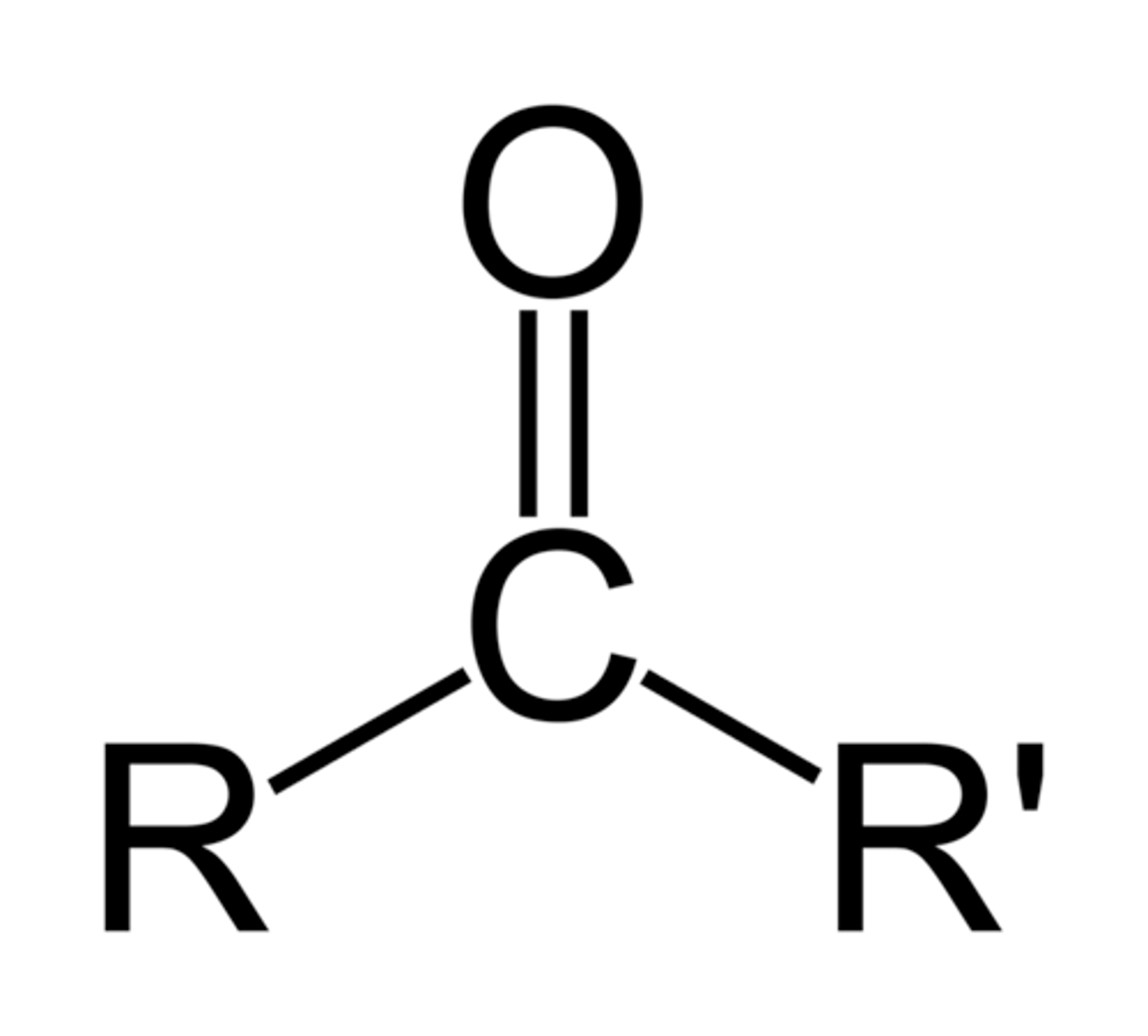
ketone
An organic compound with a carbonyl group of which the carbon atom is bonded to two other carbons (ex: acetone CH3COCH3); RCOR', suffix -one
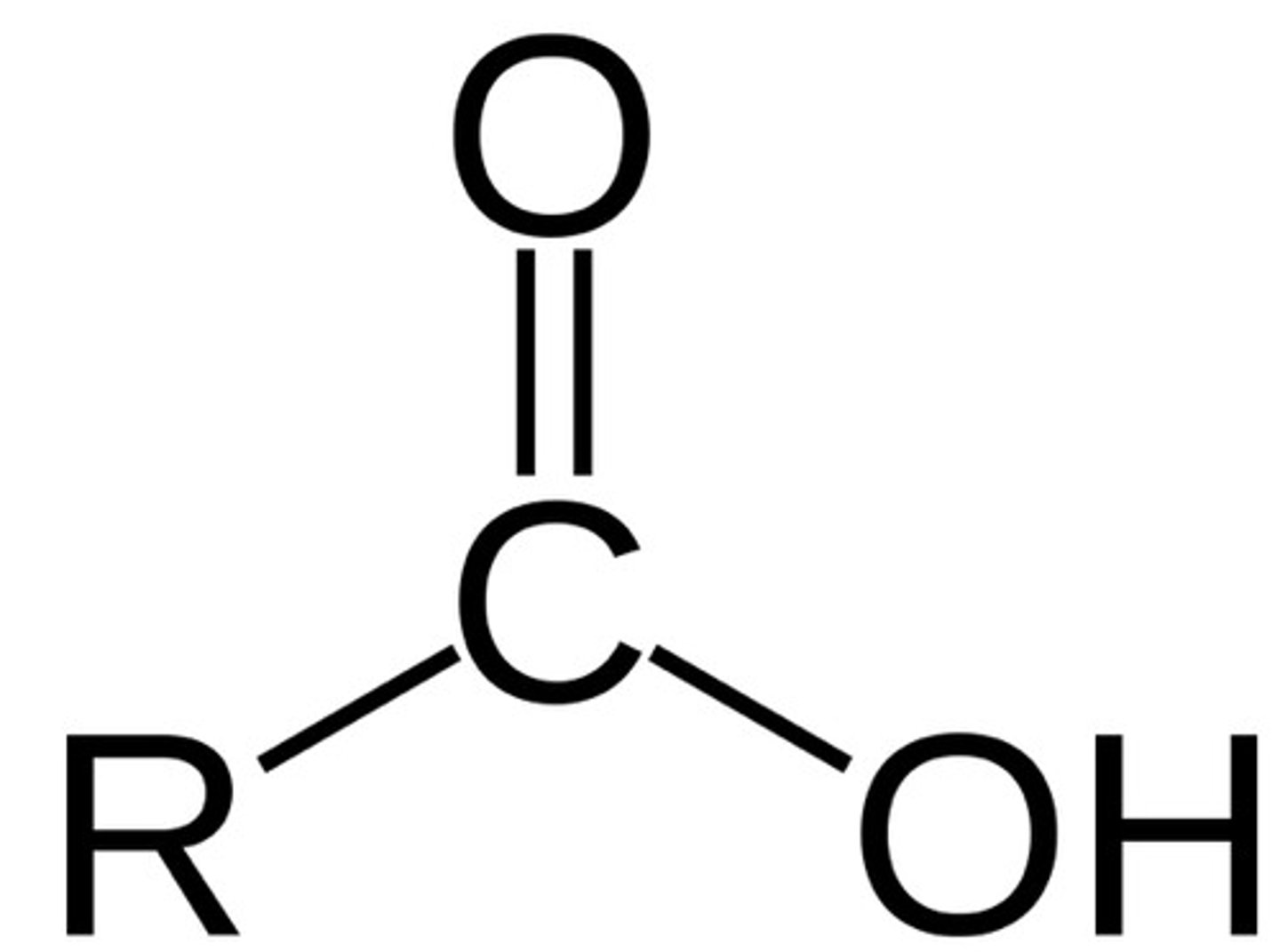
carboxylic acid
contain a carboxyl (-COOH) group (carbonyl + alcohol= carboxyl group) (ex: acetic acid CH3COOH); -RCOOH, suffix -oic acid
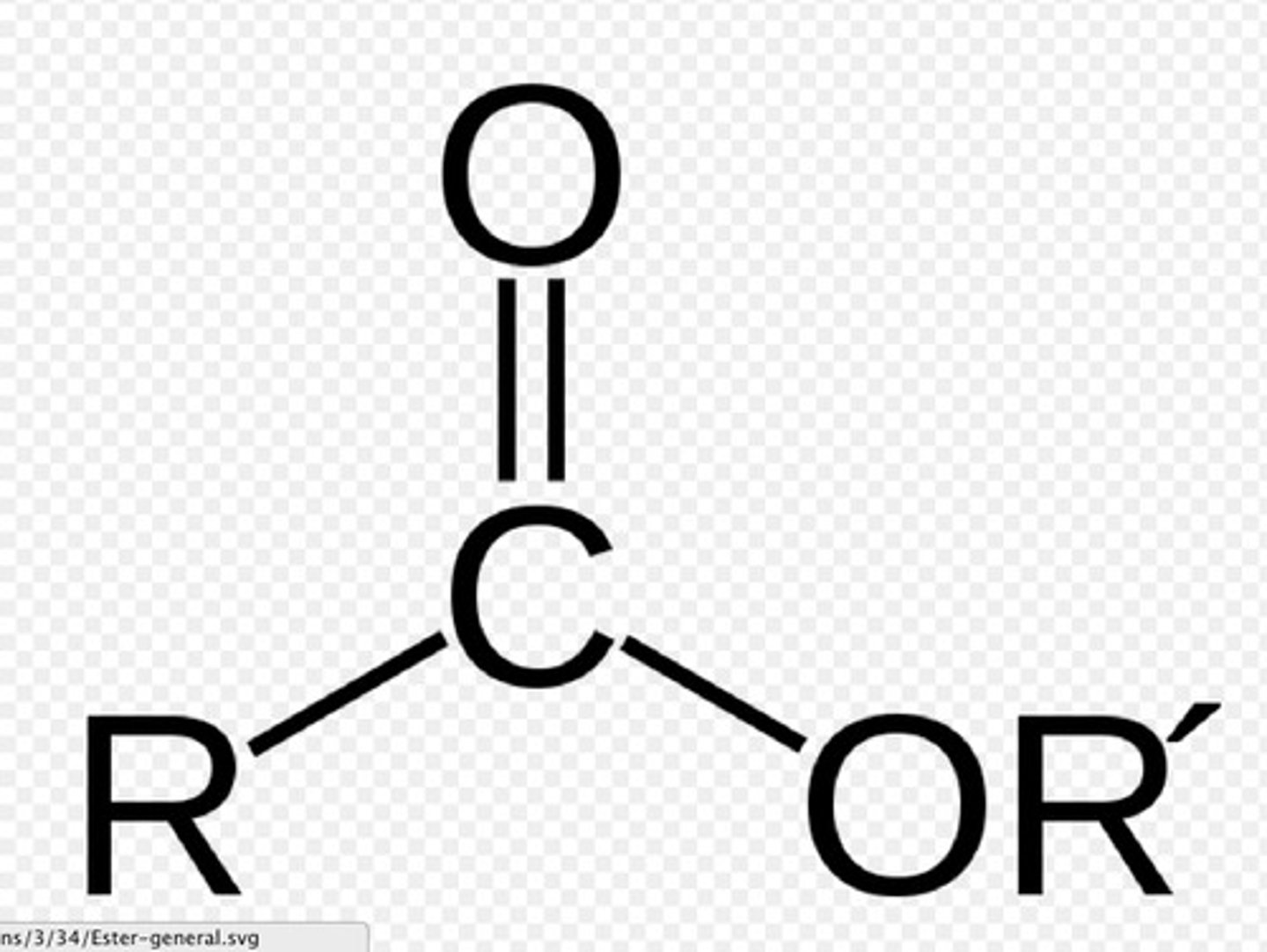
carboxylic ester
derivative of a carboxylic acid in which the carboxyl hydrogen is replaced by a carbon group (ex: ethyl-acetate); RCOOR', suffix-alkyl alkanoate
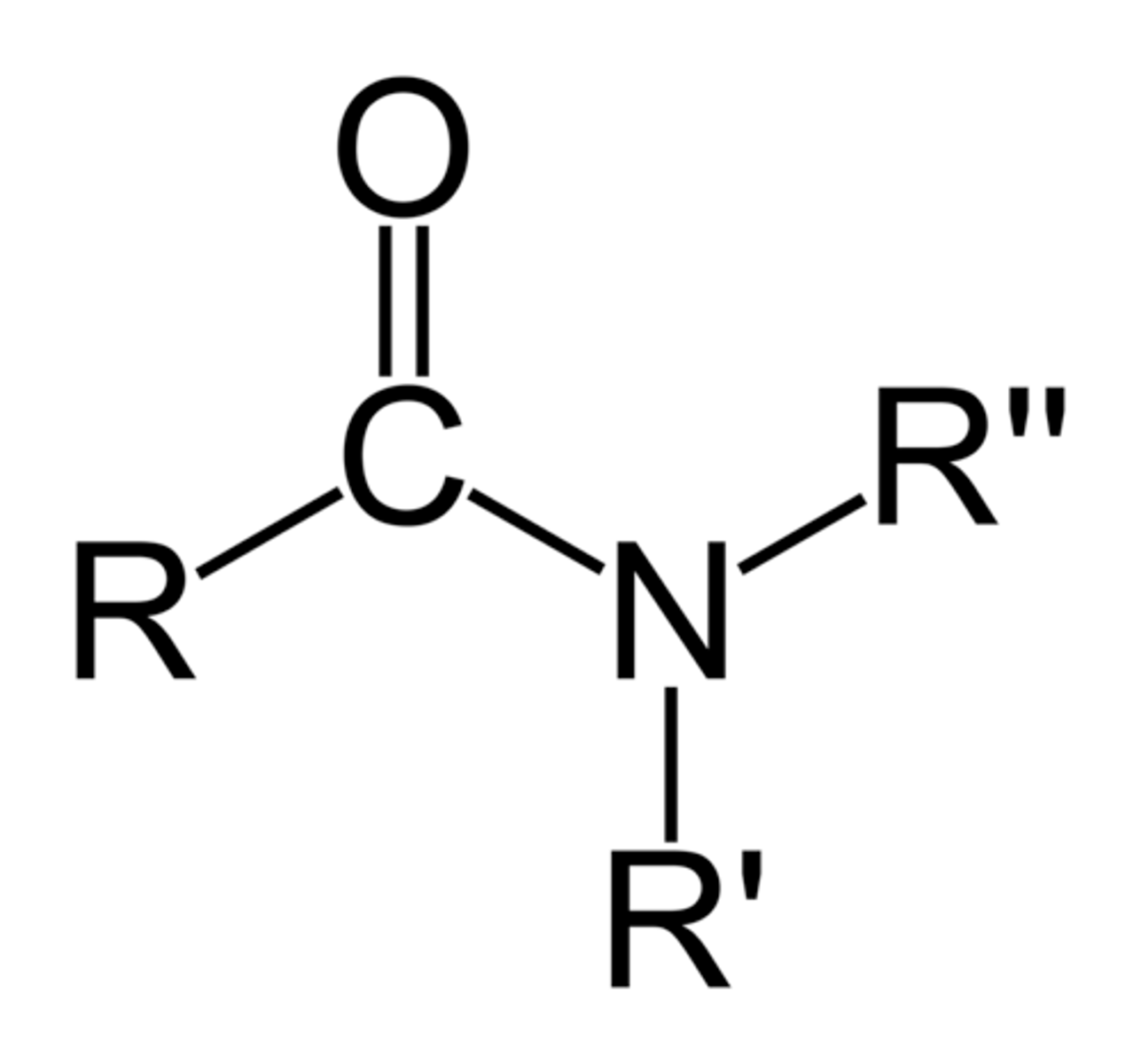
carboxylic amide
derivative of a carboxylic acid in which the OH of the -COOH group is replaced by an amine; carbonyl + amine= amide (ex: acetamide- primary amide); RCONR', suffix -amide
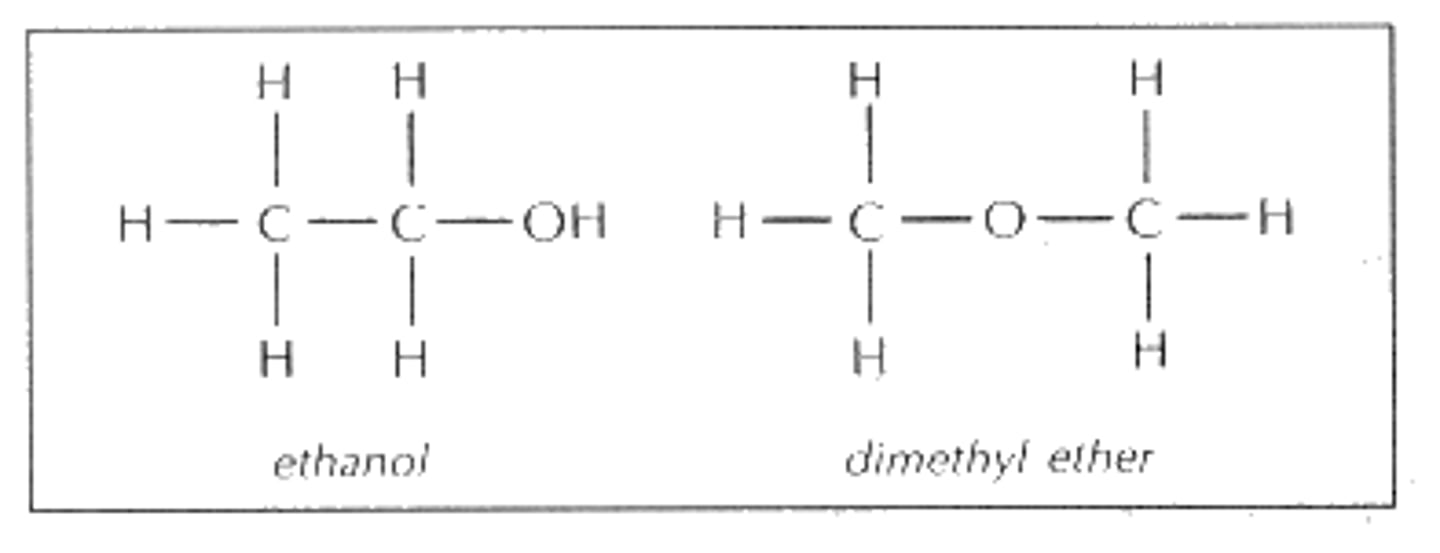
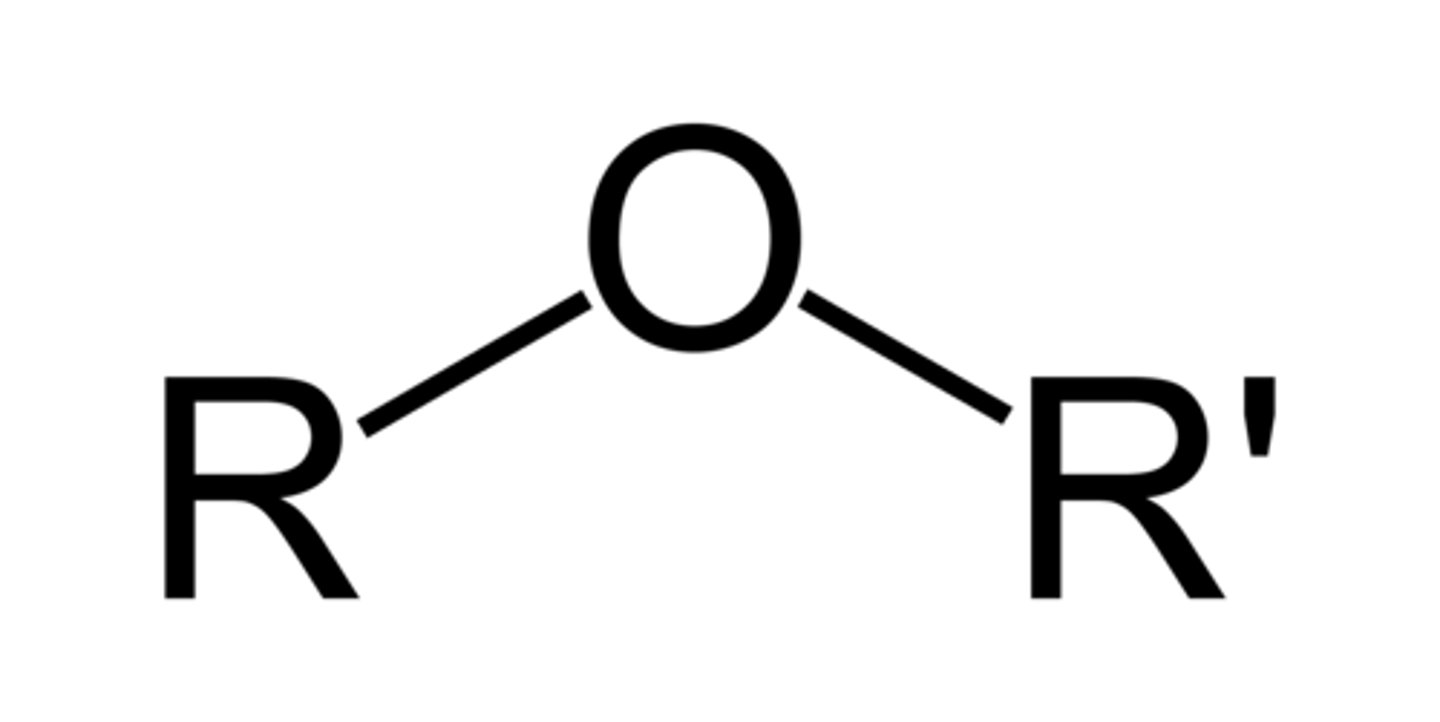
ether
oxygen bonded to two carbons by single bonds, ROR', suffix -alkyl alkyl ether
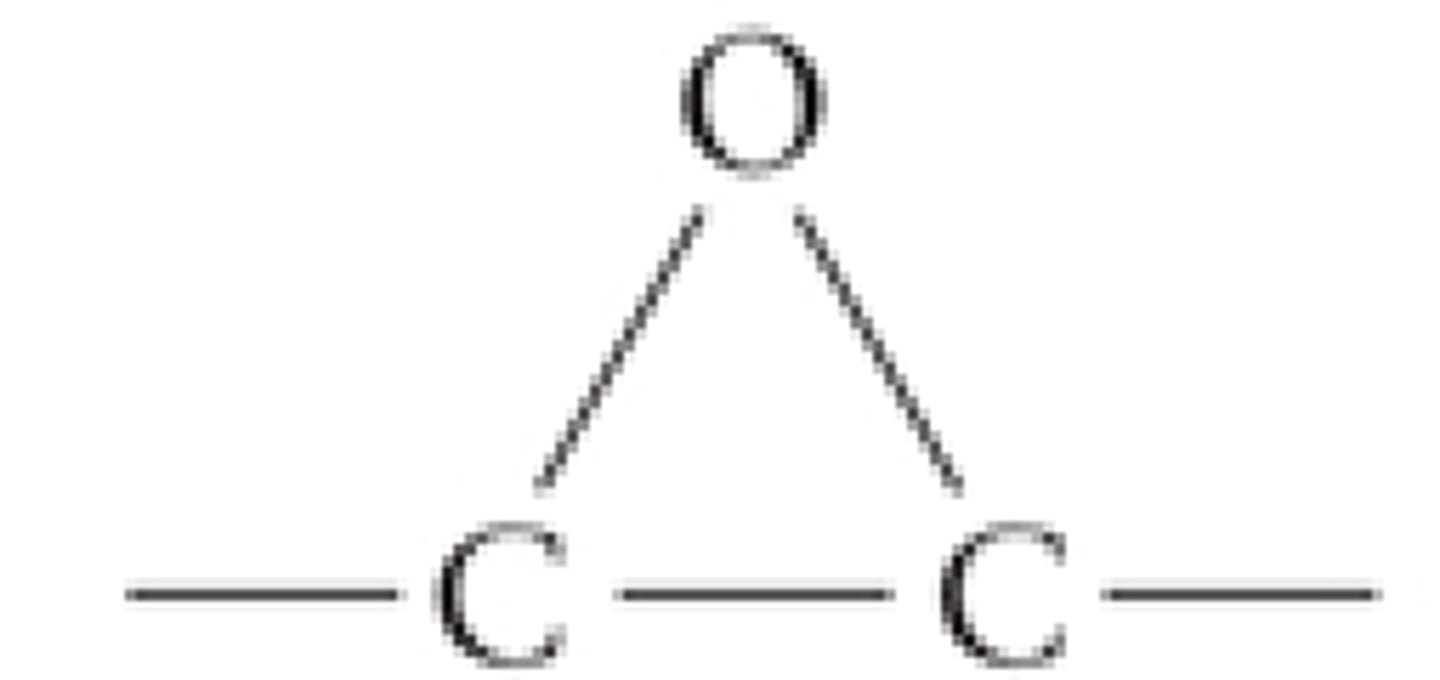
epoxy
triangle of single bonds created from an ether
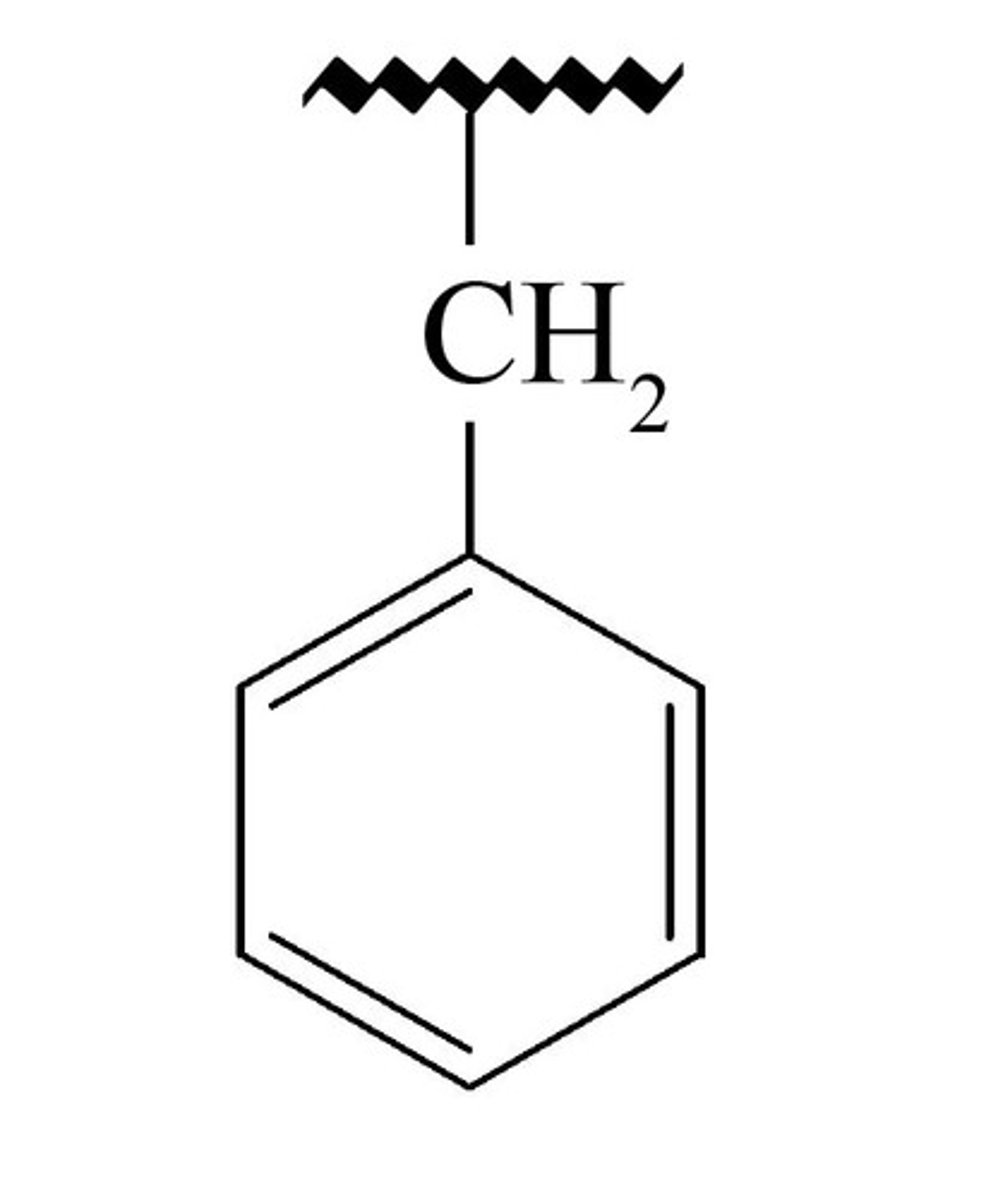
benzyl
one carbon attached to a carbon ring (ex: benzene aka cyclohexatriene)
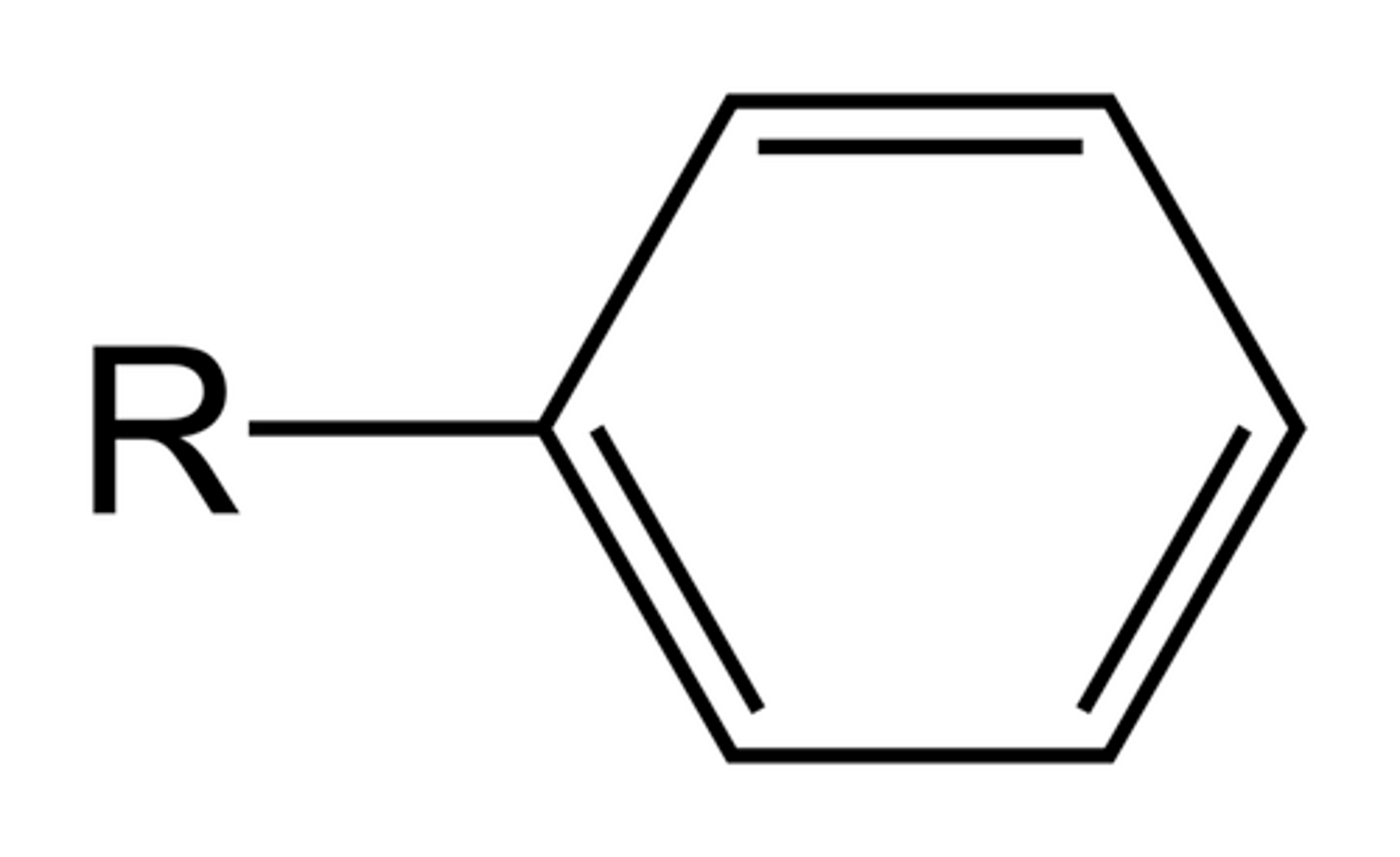
phenyl
more than one carbon attached to a carbon ring
alkyl
carbon chain attached to main carbon chain
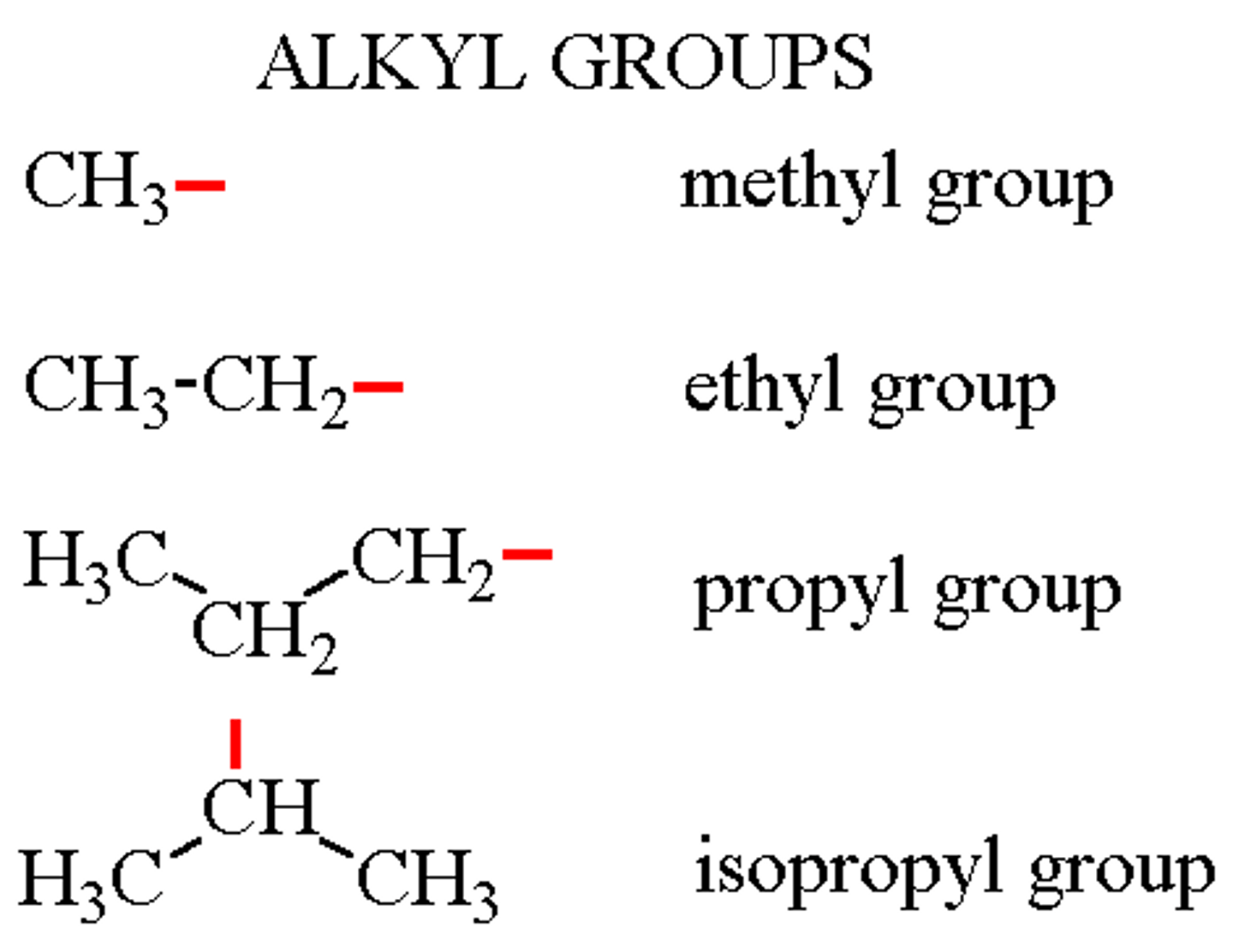
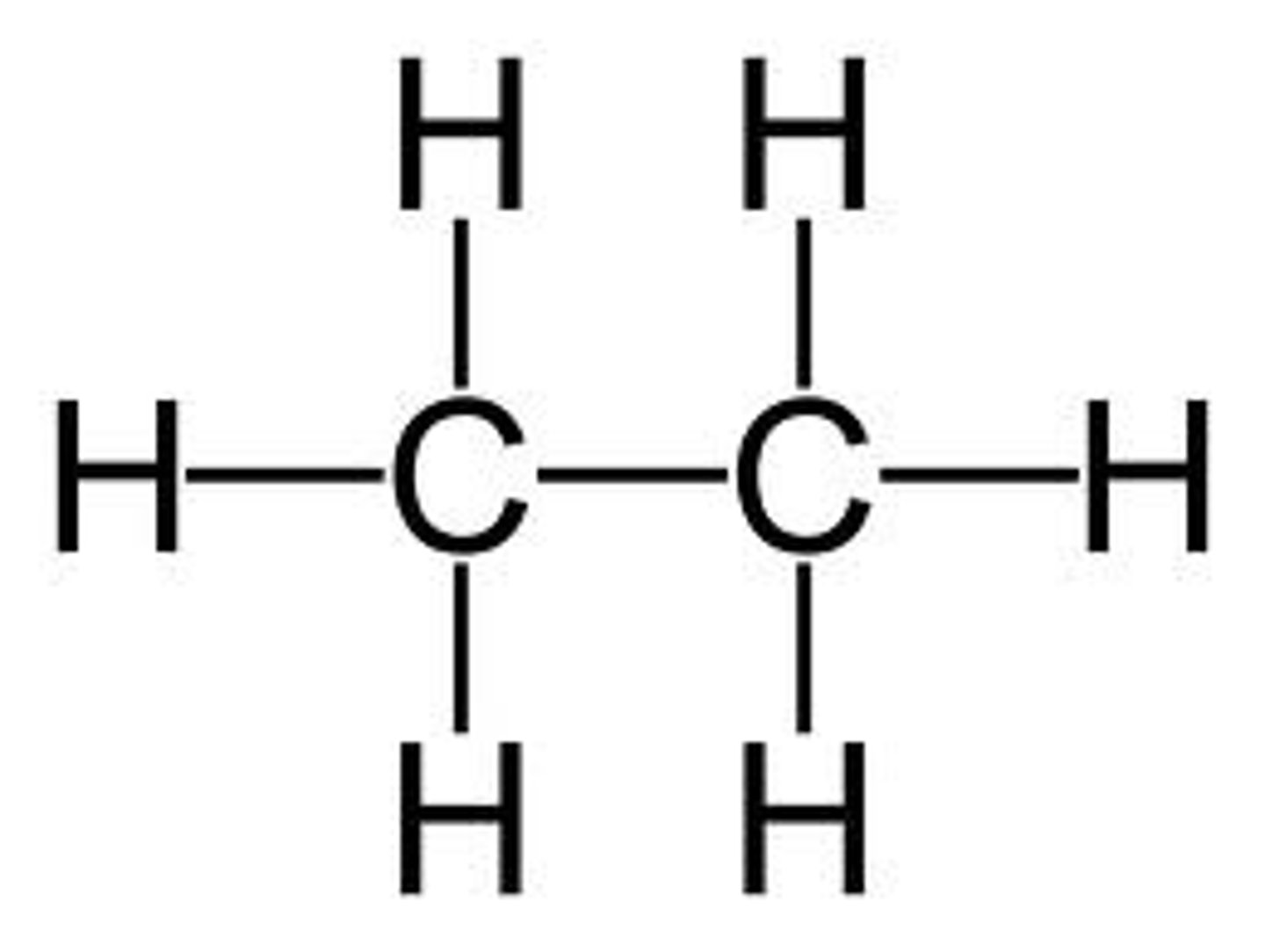
alkane
a hydrocarbon containing only single covalent bonds
alkene
A hydrocarbon that contains a double bond
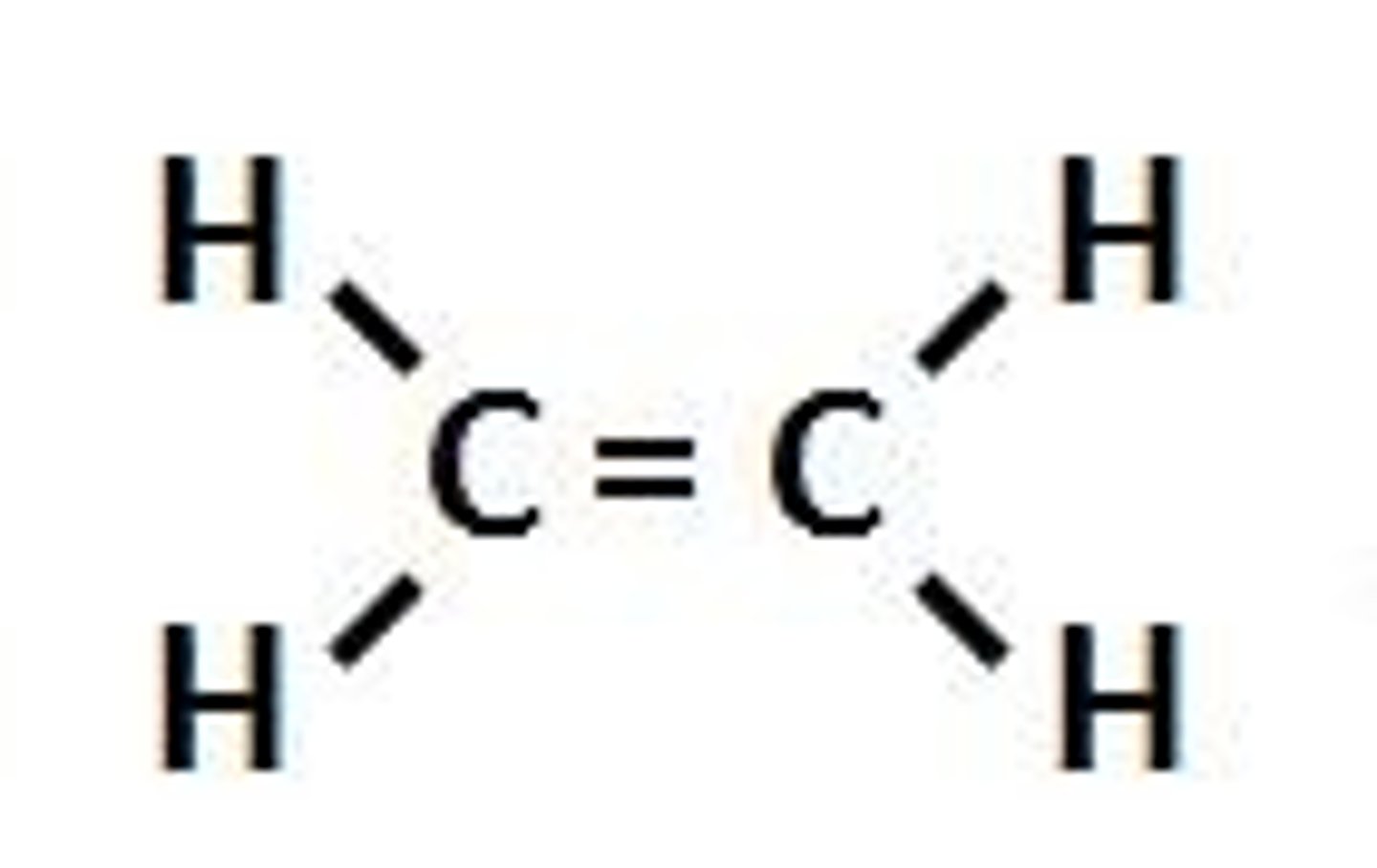
alkyne
a carbon compound with a carbon-carbon triple bond

noble gas notation
Short hand for electron configuration. Use a Noble gas to shorten the beginning of the electron configuration notation; atoms bond to have the same number of valence electrons as the noble gas nearest to its atomic number

anion
An atom that gained electrons and therefore has a negative charge. Nonmetals gain electrons
cation
atom that loses electrons and therefore has a positive charge
ionic solid
A solid consisting of positive and negative ions (cations and anions attracted to each other) arranged into crystals that are made up of regularly repeated units held together by ionic bonds
covalent bond
A chemical bond that involves sharing a pair of electrons between atoms in a molecule so they can complete their valence shells
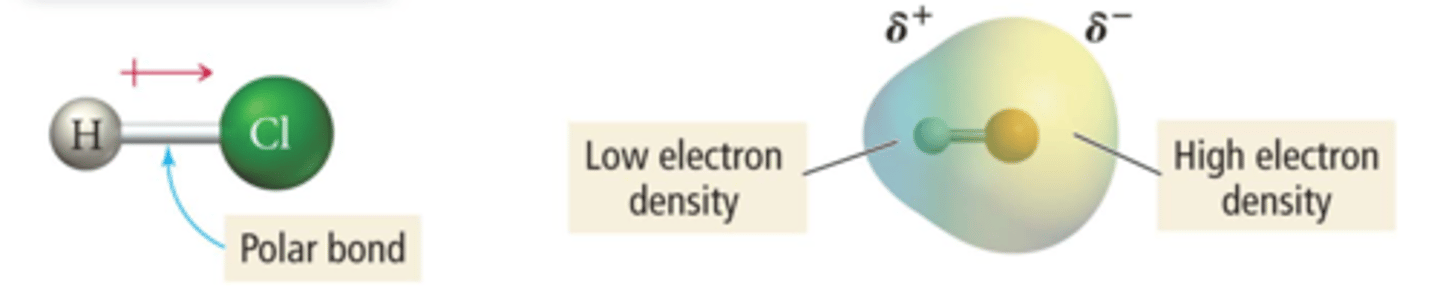
polar covalent bond
A covalent bond between atoms that differ in electronegativity--> has a dipole moment. The shared electrons are pulled closer to the more electronegative atom, making it slightly negative and the other atom slightly positive. partially ionic and partially covalent
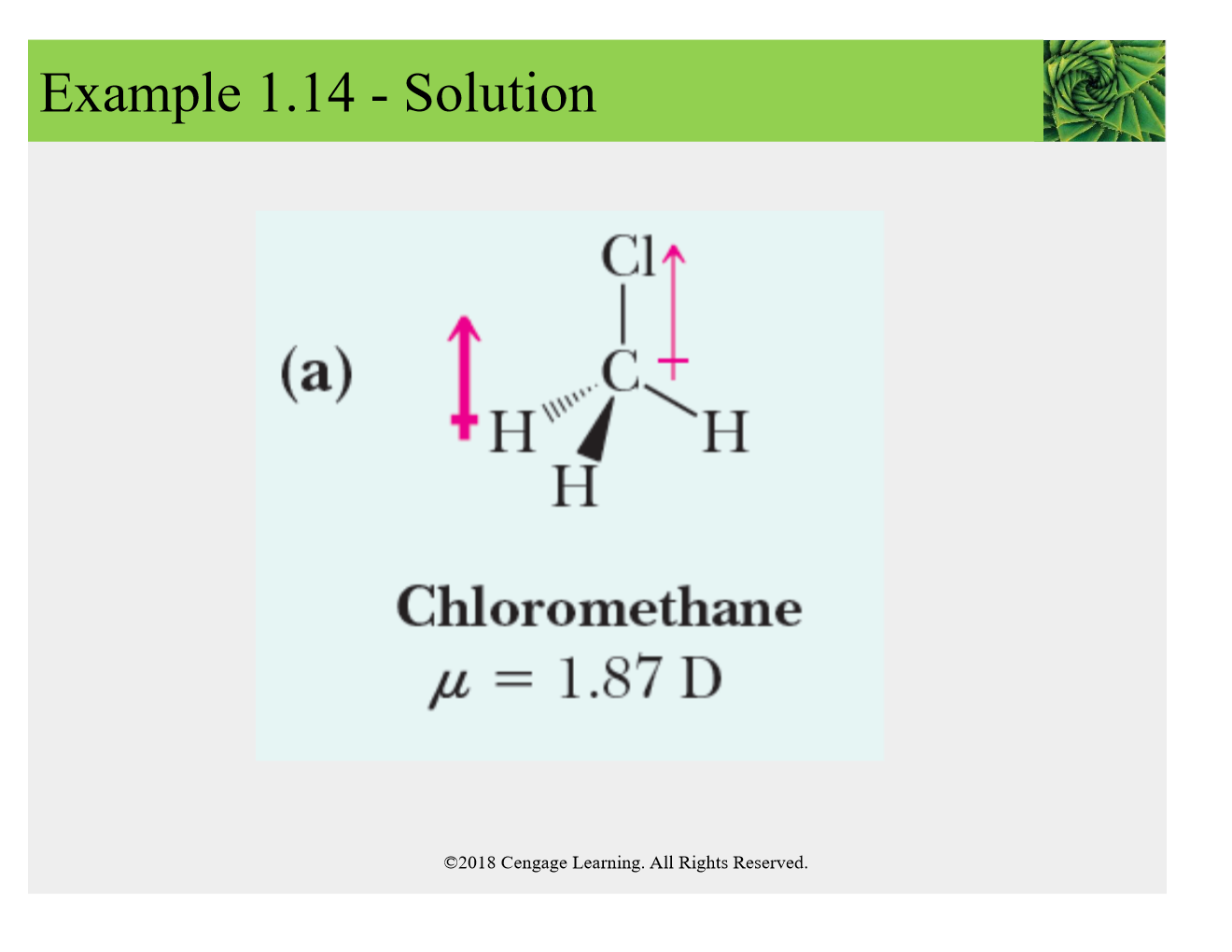
ex: H-Cl diff in EN--> 3.0-2.1= .9, arrow points from positvie end (lower case delta +) to the negative end (lower case delta -)
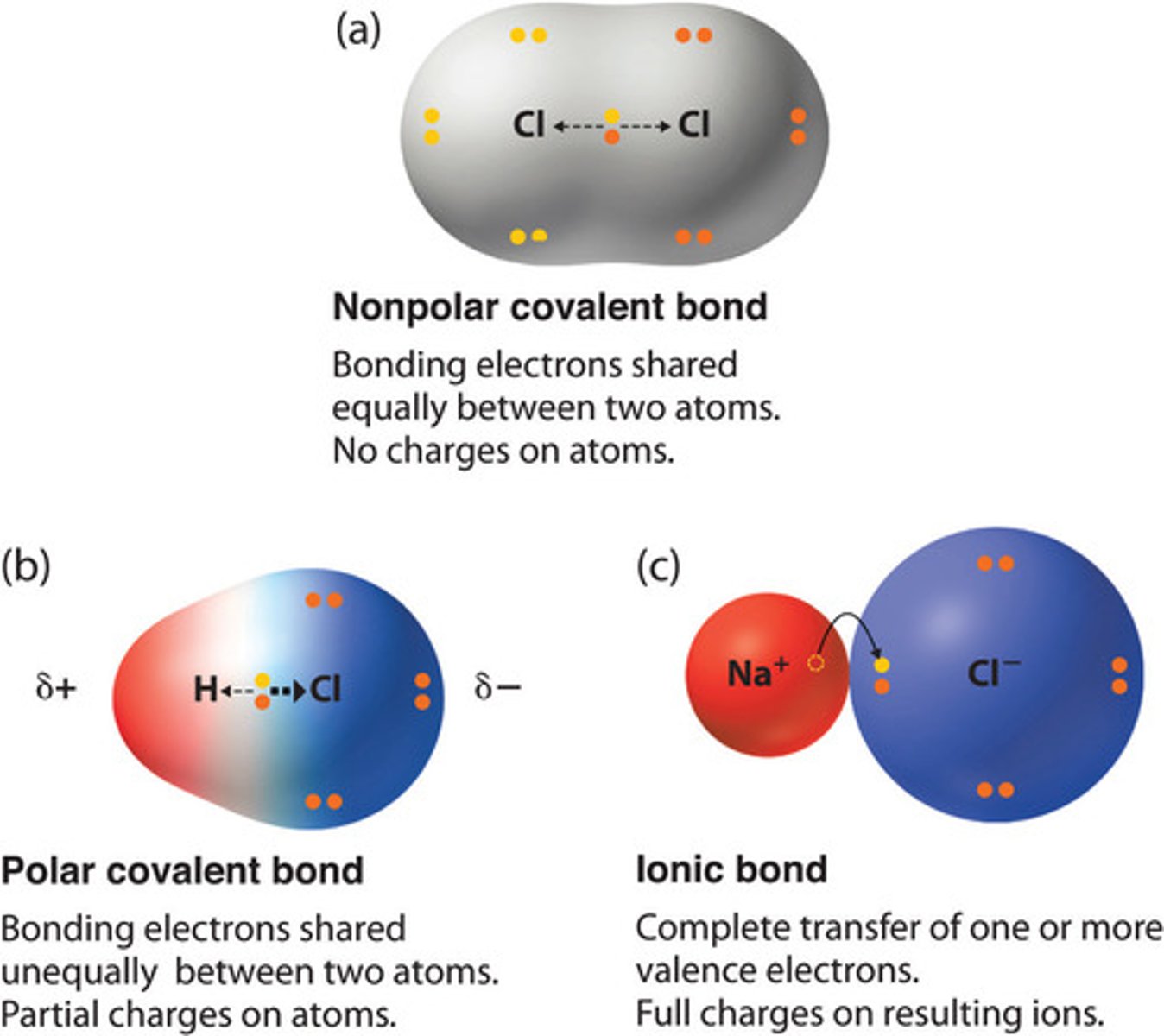
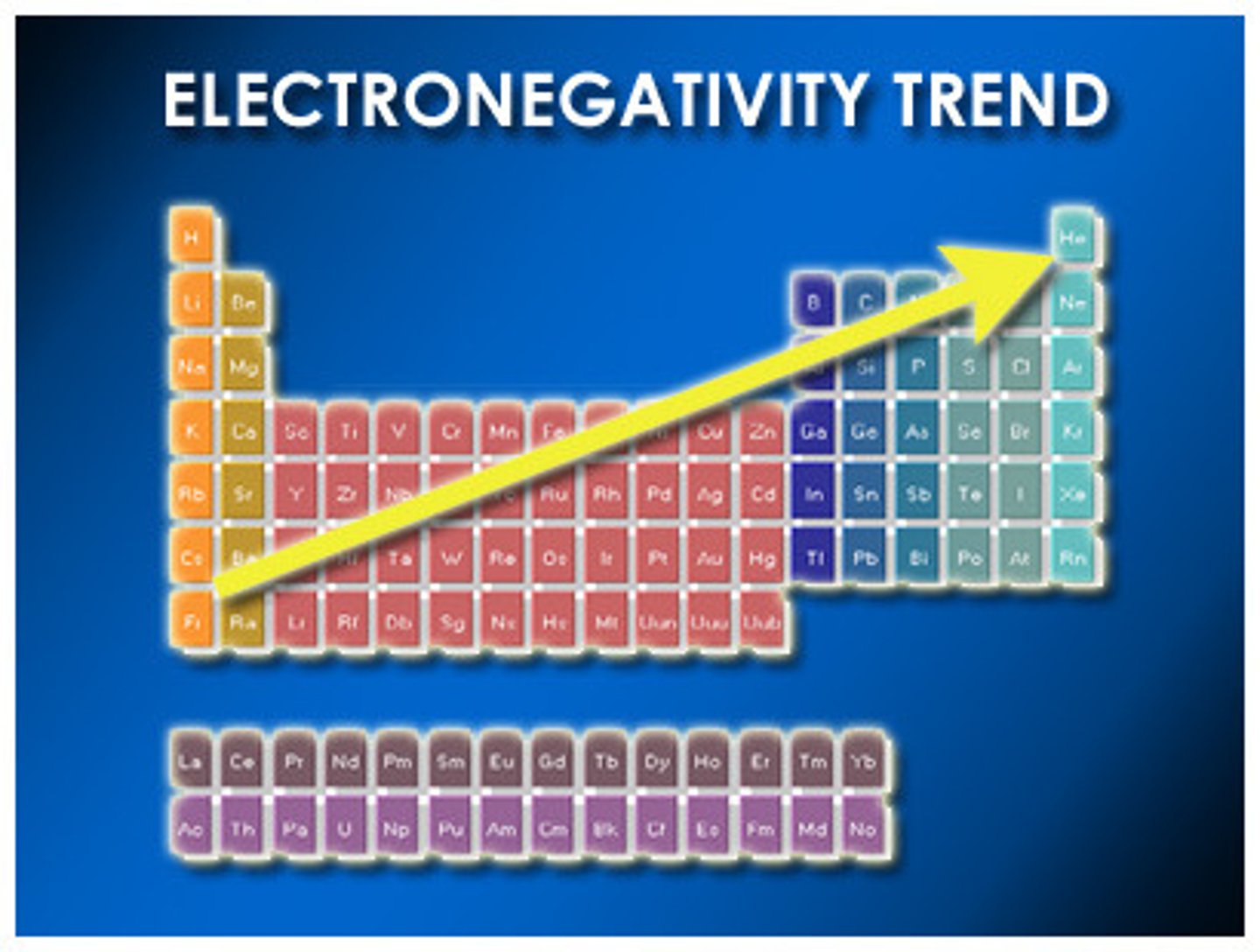
electronegativity
A measure of the ability of an atom to attract electrons it shares with another atom in a chemical bond
Pauling scale of electronegativity
Generally increases from left to right in a row and from bottom to top in a column
difference in electronegativity for ionic bond
greater than 1.9
difference in electronegativity for covalent bond
less than 1.9, greater than .3
difference in electronegativity for nonpolar covalent bond
less than .3
simplest covalent bond
H2; electrons shared between both atoms and also simultaneously fills valence shells of both atoms
single covalent bond
1 shared pair of electrons where s orbitals overlap to create bond
double covalent bond
2 shared pairs of electrons where two s orbitals overlap to create sigma bond and two p orbitals overlap to create pi bond
triple covalent
3 shared pairs of electrons where two s orbitals overlap to create sigma bond and four p orbitals overlap to create two pi bonds
dipole moment
occurs when a molecule has some charge separation (usually because the molecule is polar); more polar bond--> larger electronegativity difference--> larger dipole moment. Product of the amount of partial charge at either end of a molecule's dipole multiplied by the distance between them, given equation "p=qd". P is the dipole moment, q is the partial charge, and d is the distance separating the dipole

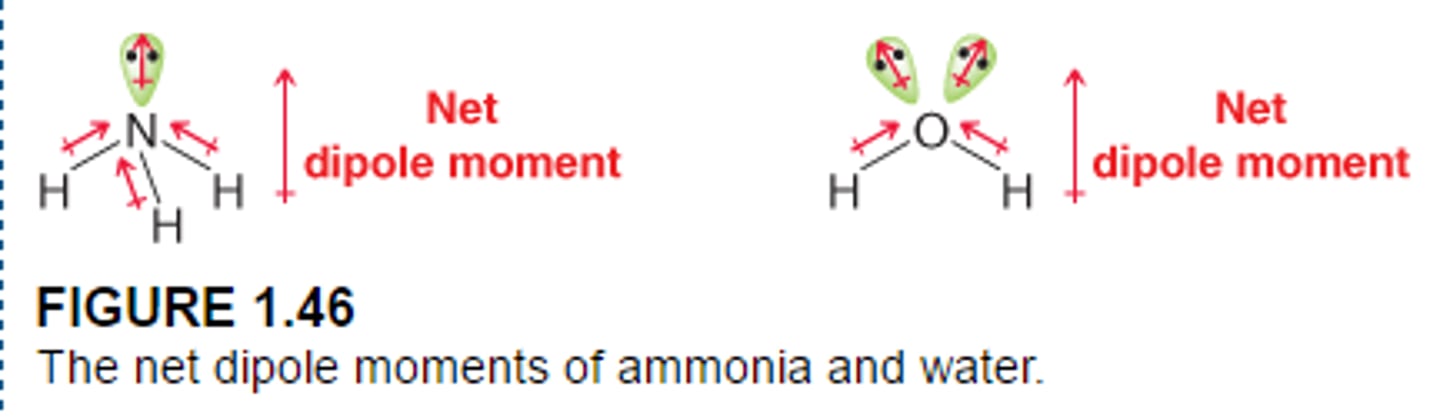
molecular dipole moment
vector sum of individual bond dipole moments in a molecule (reported in debyes D)
formation of ions
ions form if the difference in electronegativity between the atoms is greater than 1.9
ex: Na (.9 EN) and F (4.0 EN)
curved, single headed arrow shows transfer f one electron from Na (that was a single 3s) to the partially filled valence shell of F to form Na+F-
carbanion
a compound in which the carbon atom bears a negative charge (3 bonds and one lone pair) where carbon usually has 4 bonds and 0 lone pairs
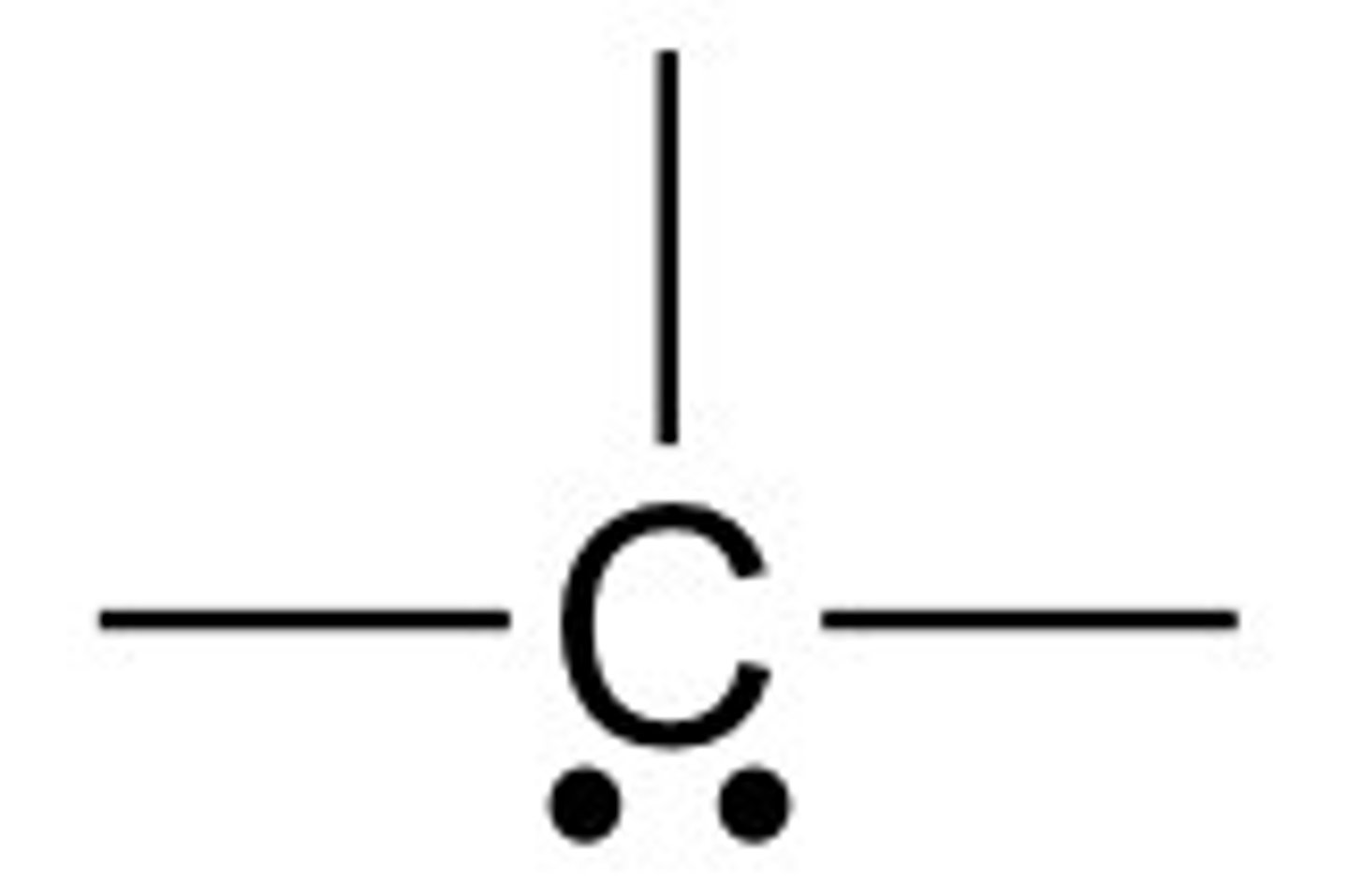
nitrogen in bonding
usually has 3 bonds and one lone pair
halogens in bonding
usually have 1 bond and three lone pairs
boron in bonding
satisfied with 3 bonds
formal charge
Charge assigned to an atom in a molecule or polyatomic ion, calculated by (# valence electrons) - [(# 1/2 bonding electrons aka shared electrons) + (# nonbonding electrons aka unshared electrons)]. Molecules containing atoms with lower formal charges tend to be more stable than those with higher formal charges. sum of all formal charges is the total charge on a molecule or ion
elements that can form double or triple bonds
SPONC
expanded octet
An exception to the octet rule that permits atoms in the third row or lower on the periodic table to have more than eight electrons in a Lewis structure; if element is after aluminum on PT
noble gases
Elements in group 8A of the periodic table. Have no charge and are gases under normal conditions. (Helium, Neon, Argon, Krypton, Xenon, Radon); bottom, larger noble gases at bottom of PT can make bonds
elements satisfied with less than 8 electrons in bonding
molecules that contain elements in group 3A and Boron and aluminum
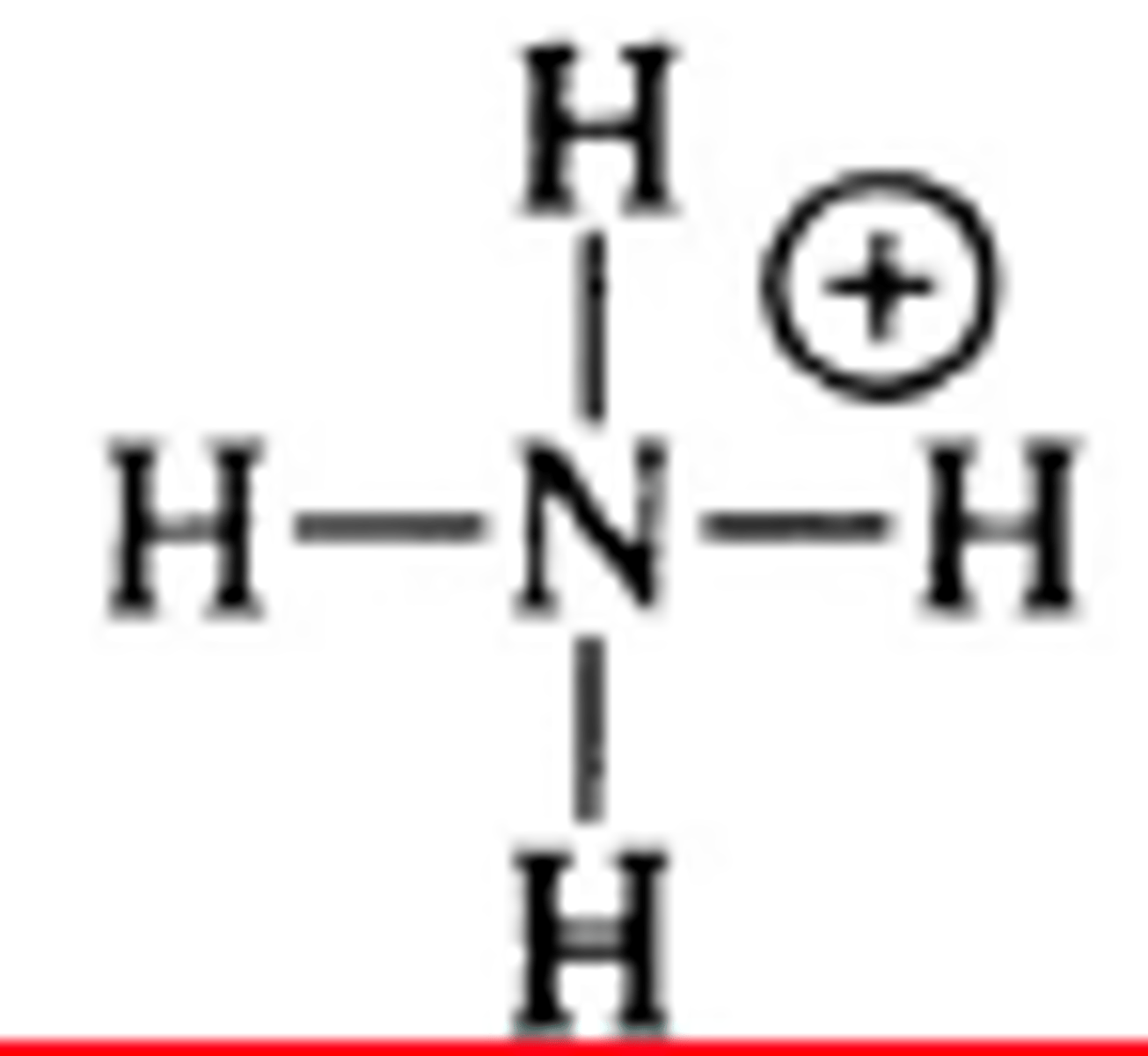
NH4+
PCl3
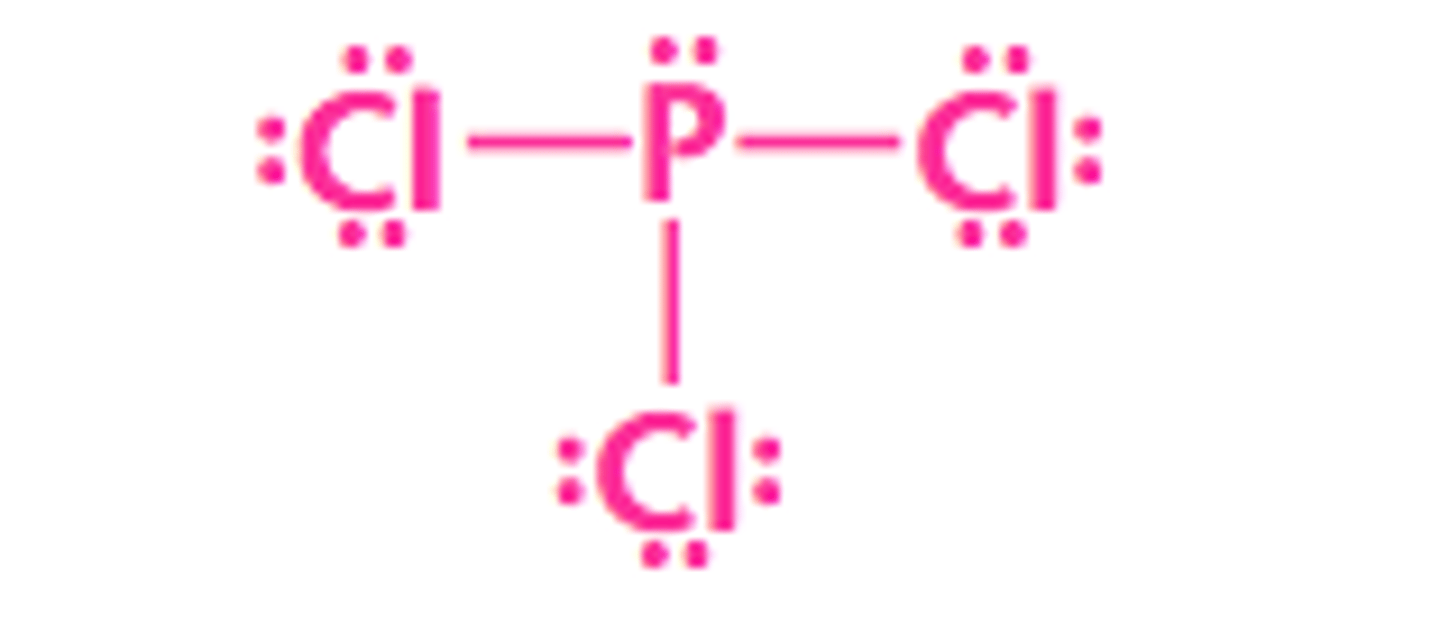
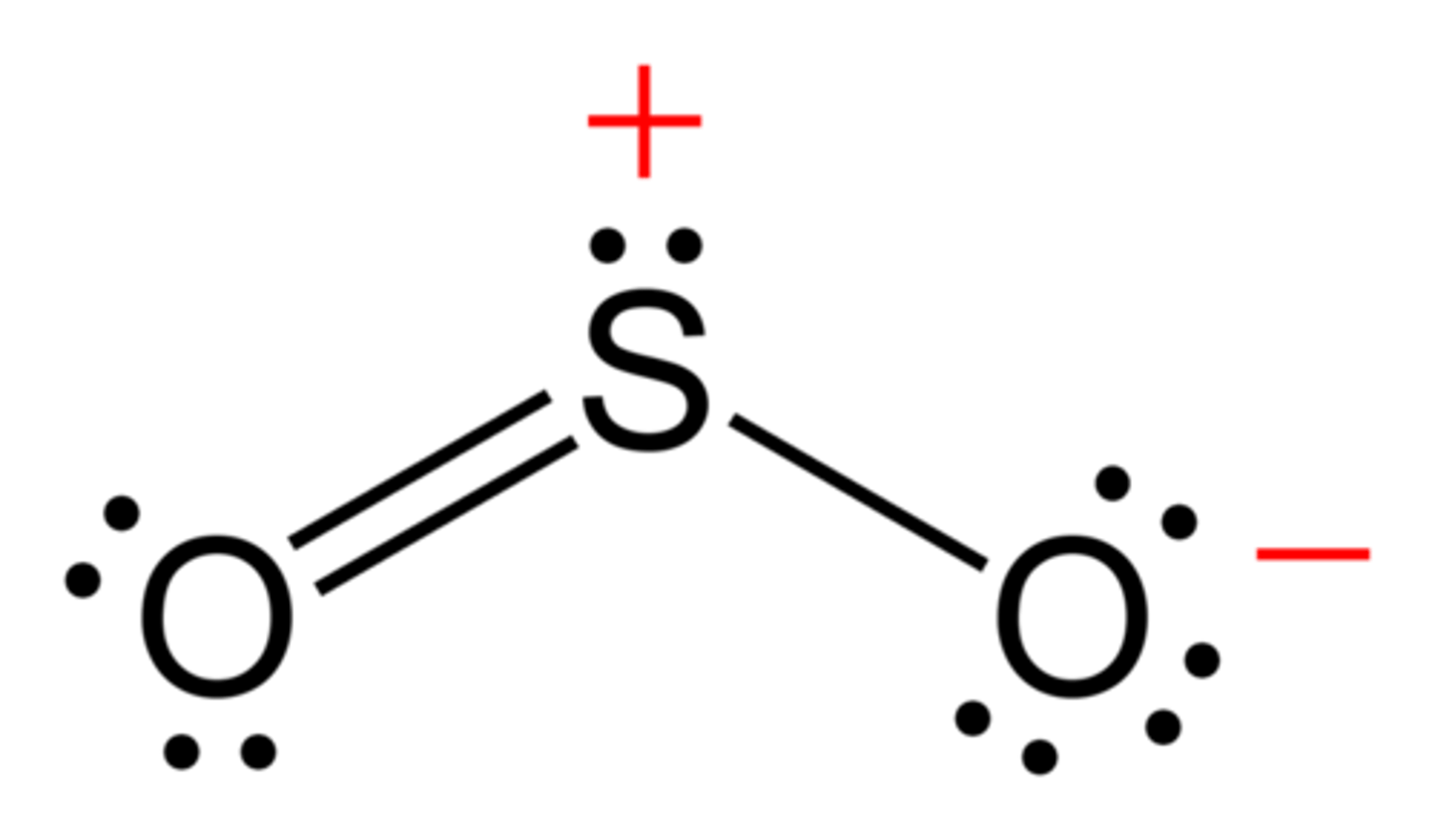
SO2
XeF2

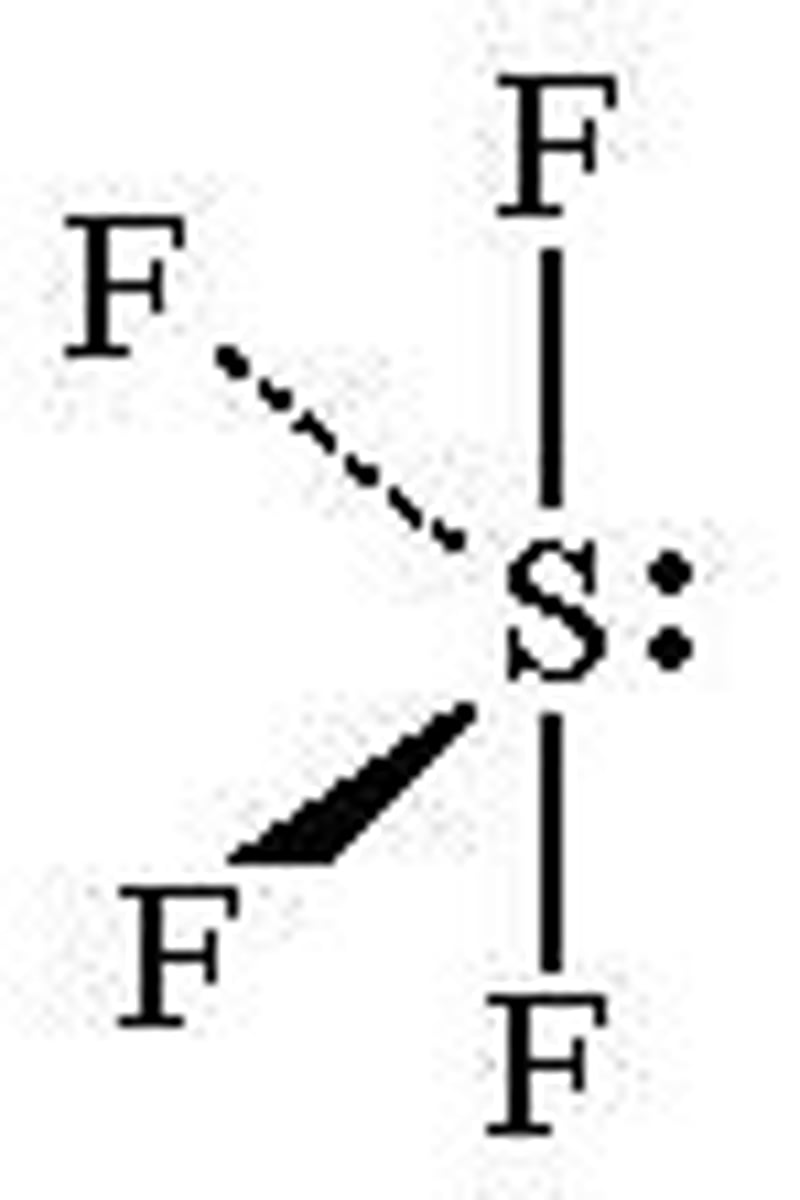
SF4
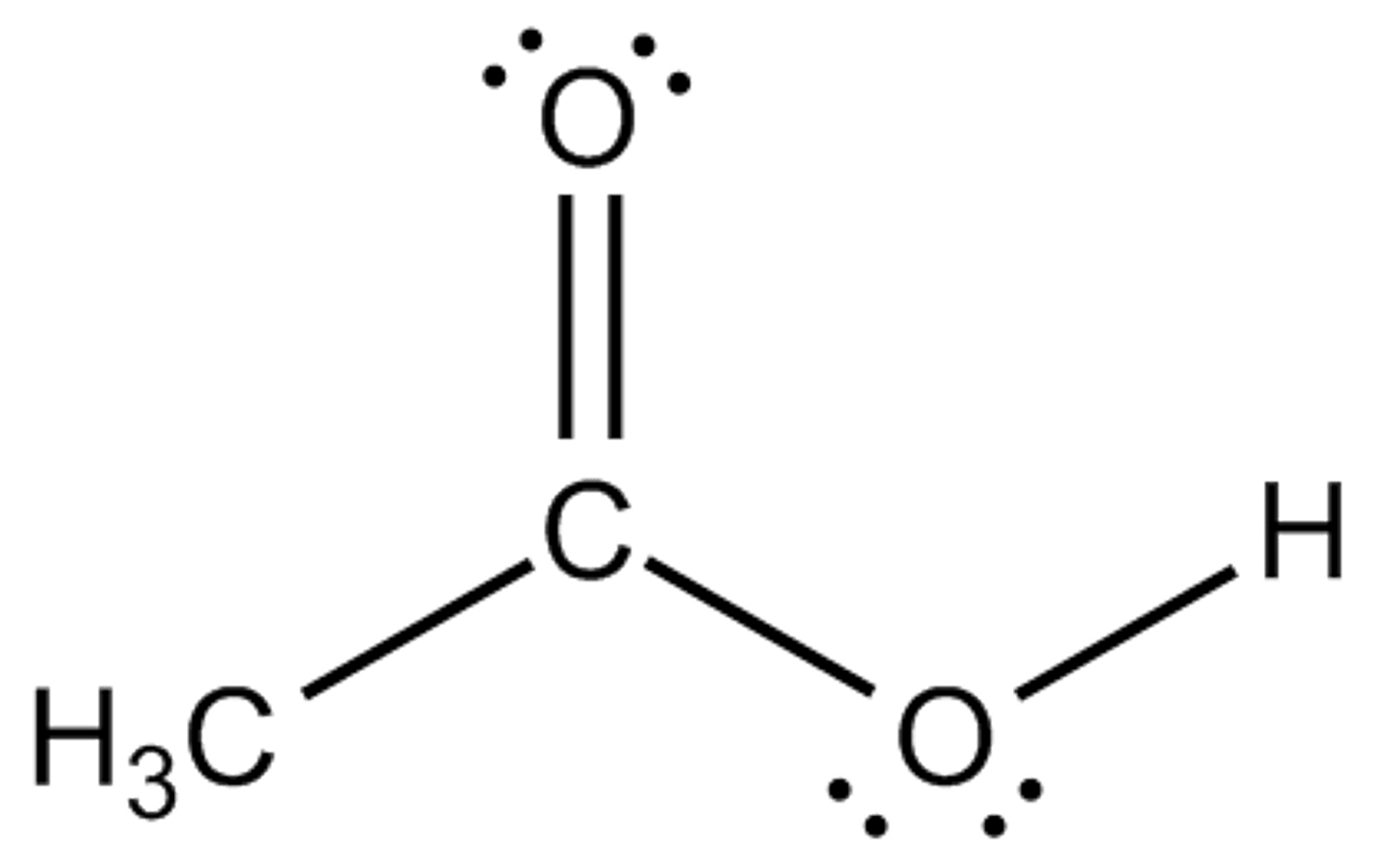
CH3COOH
HNO3

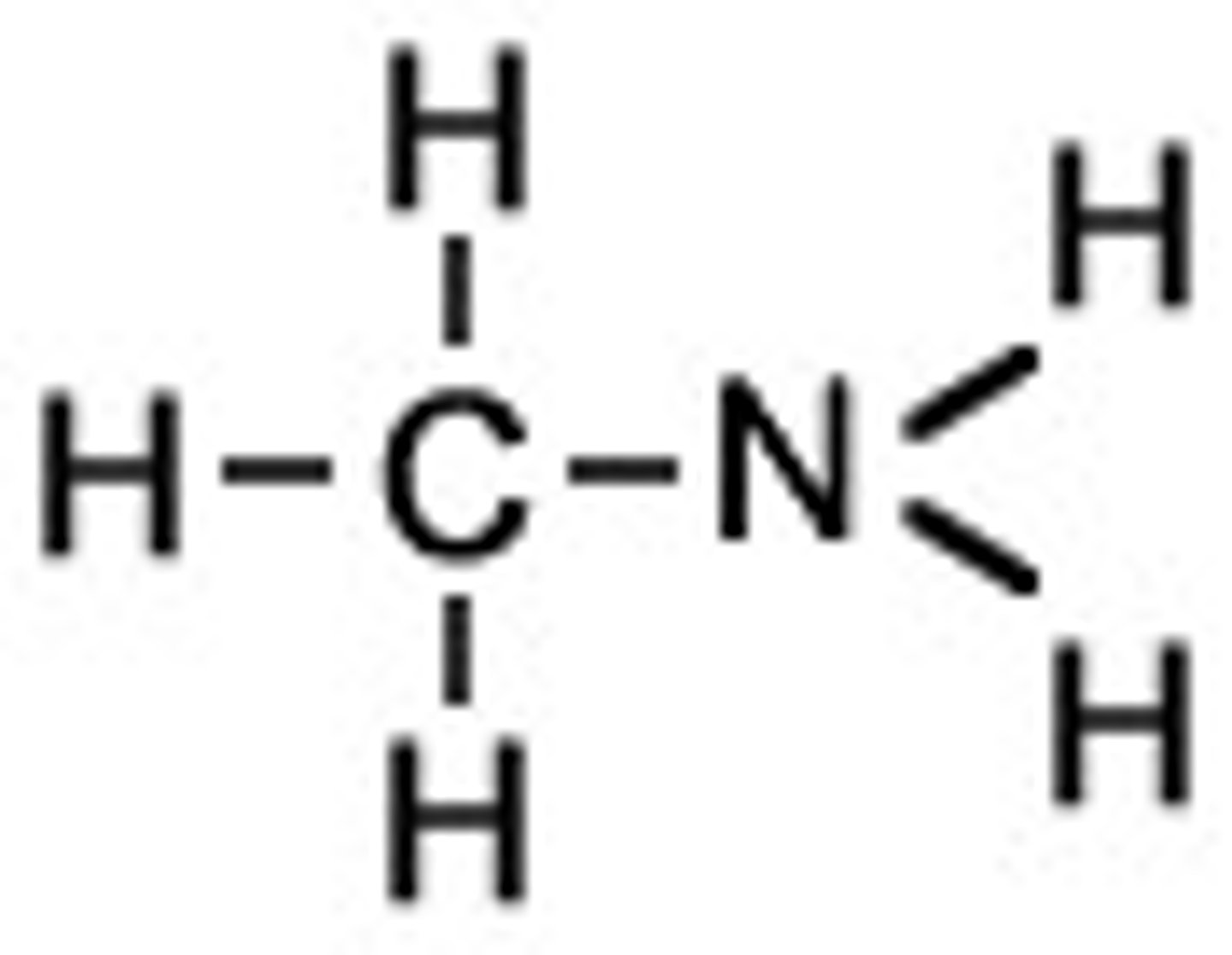
CH3NH2
one lone pair on nitrogen
contributing structures
(Linus Pauling) many molecules and ions are best described by writing two or more lewis structures; individual lewis structures are called contributing structures; connect individual contributing structures by double-headed resonance arrows, molecule or ion is a hybrid of various contributing structures
electron pushing diagram
The use of curved arrows to show the redistribution of valence electrons in various contributing structures
minimizing formal charge
expanded octet sometimes preferred to minimize formal charge; more bonds--> lower energy--> more stable
steric number
total number of bonds and lone pairs on central atom (double and triple bond count as one electron region density, not multiple)
More viable contributing structures
-no separation of charge--> more stable
-more covalent bonds--> more stable
-filled valence shell--> more viable
-negative charge on a more electronegative atom--> more viable
EX: CH3COCH3
valence shell electron pair repulsion
atoms surrounded by regions of electron density that
repel each other and arrange themselves around the central atom so that they are as far apart from eachother as possible
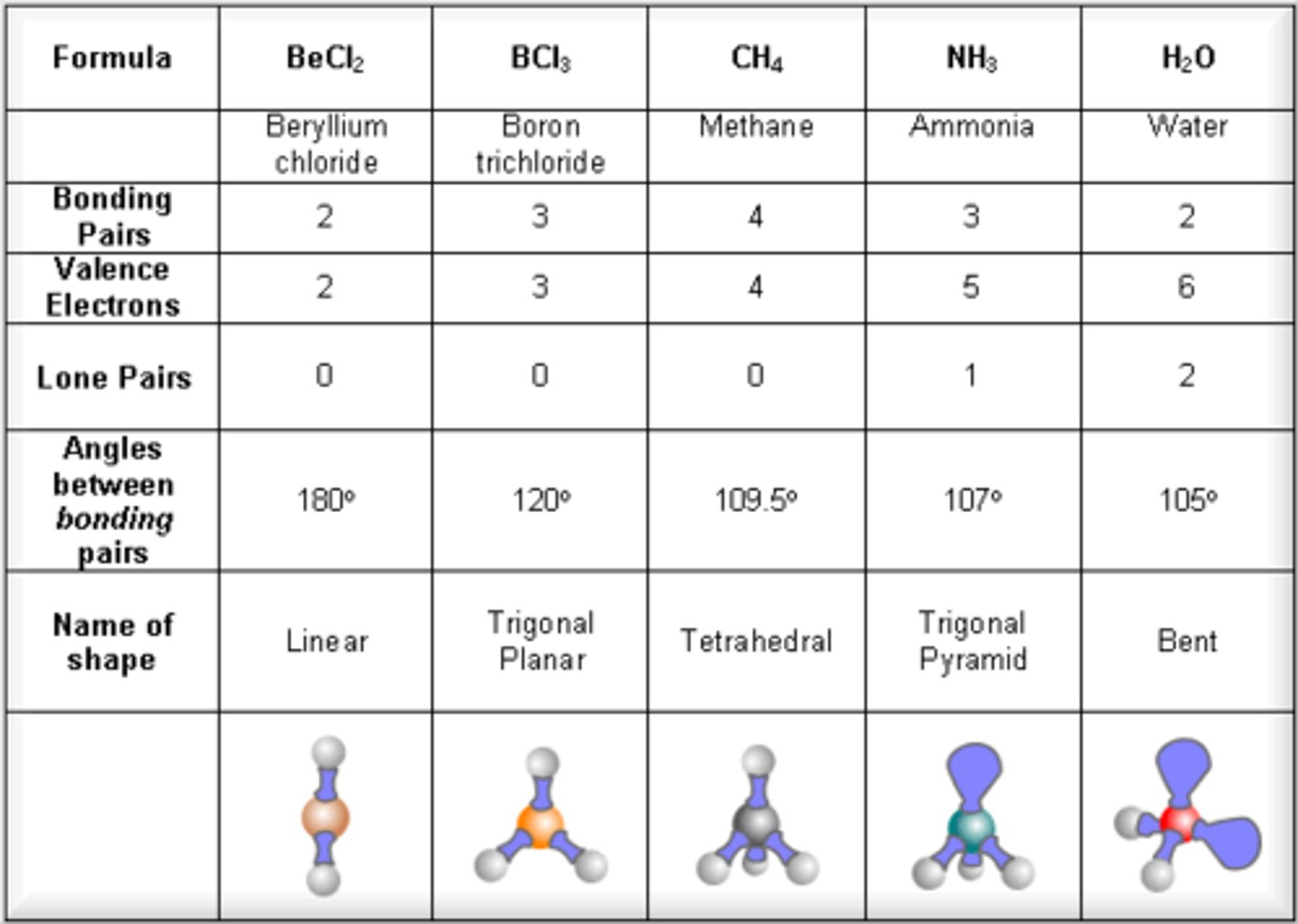
electron geometry- 2 regions of electron density
linear, bond angles= 180
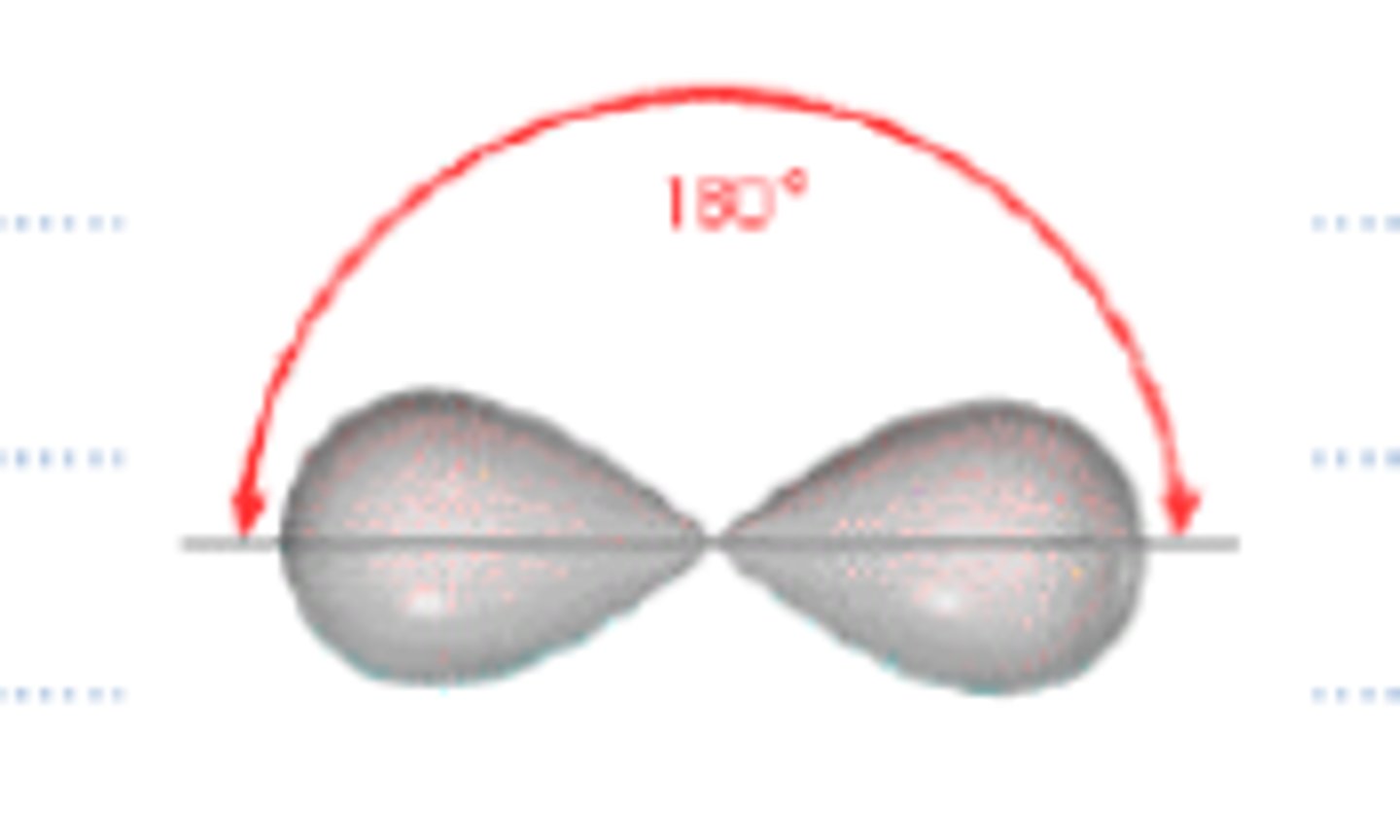
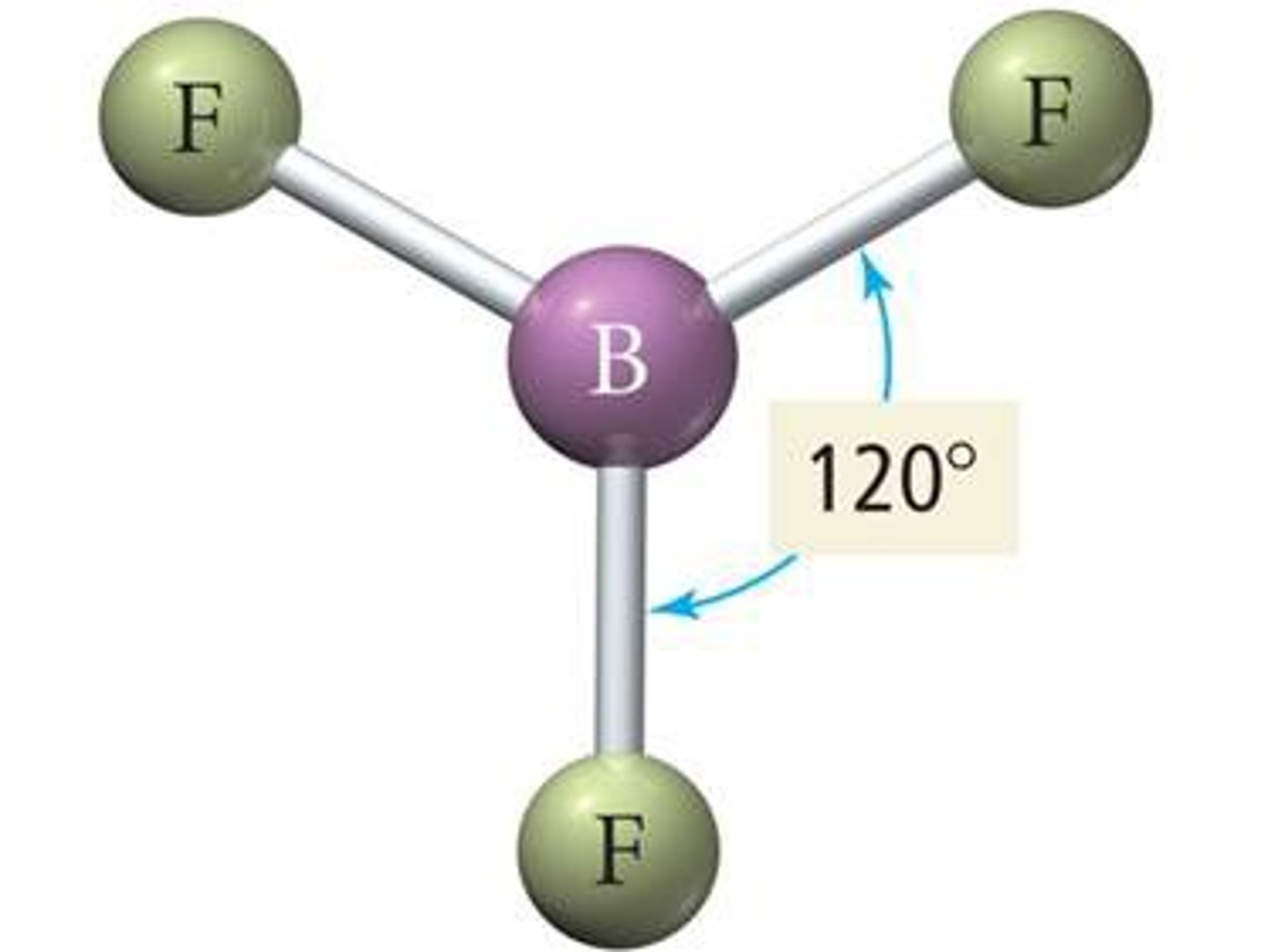
electron geometry- 3 regions of electron density
trigonal planar, bond angles = 120
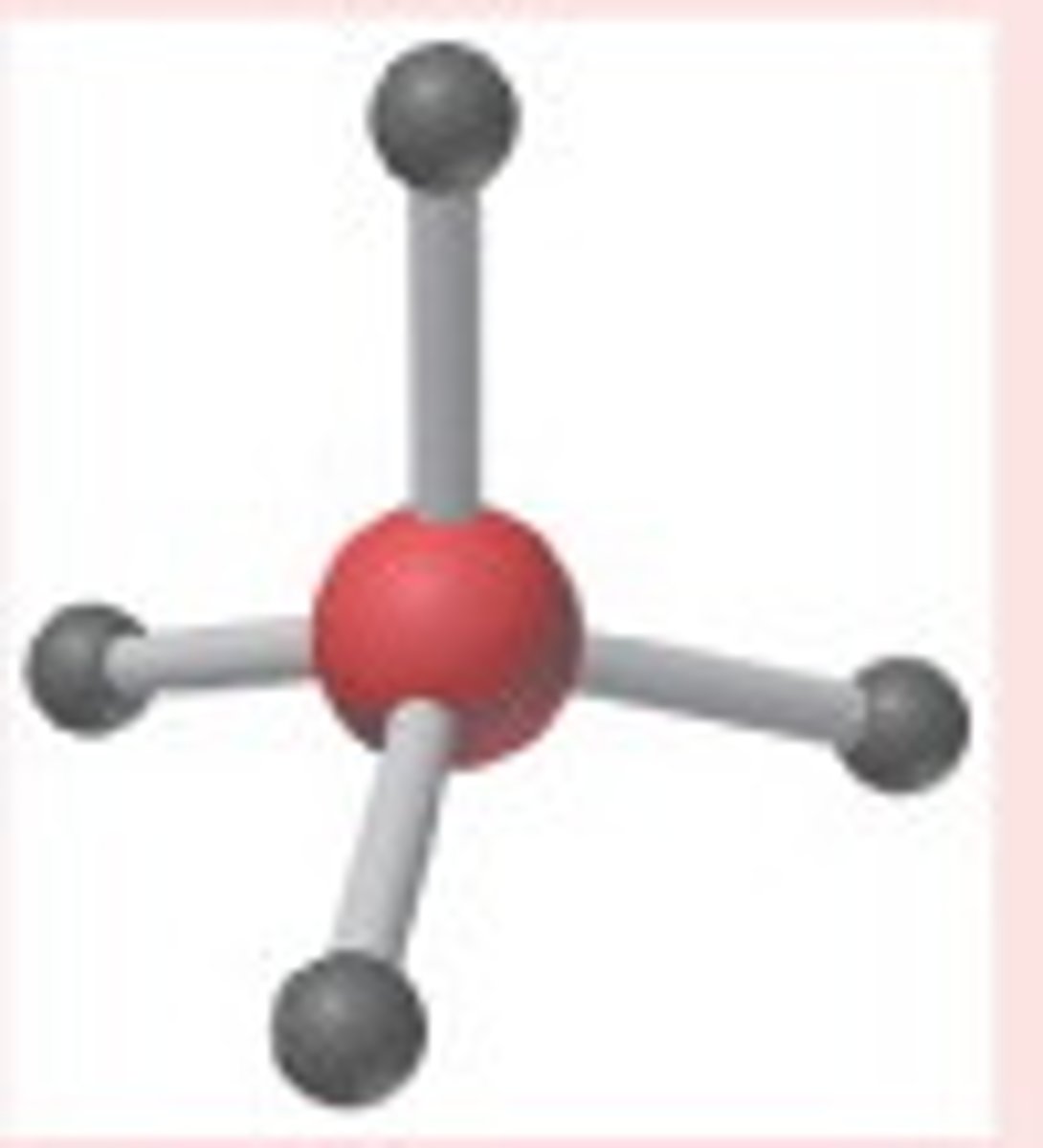
electron geometry- 4 regions of electron density
tetrahedral, bond angles= 109.5
molecular geometry- 2 bonds and 0 lone pairs
linear (180)
molecular geometry- 2 bonds and 1 lone pairs
bent (or angular) (120)
molecular geometry- 3 bonds and 0 lone pairs
trigonal planar
molecular geometry- 3 bonds and 1 lone pairs
trigonal pyrimidal/ squatty pyramid (107)
molecular geometry- 2 bonds and 2 lone pairs
bent (180)
molecular geometry- 4 bonds and 0 lone pairs
tetrahedral (109.5)
polar molecules
1. determine if molecule has polar bonds
2. determine arrangement of bonds
- some molecules have polar bonds but no molecular dipole moment--> nonpolar molecules (ex: CO2, BF3, CCl4)
-some molecules have polar bonds and are polar molecules (ex: H2O, NH3 ammonia, C2O formaldehyde)
electrostatic potential (elpot) maps
electron density representation/ distribution; increased electron density, more likely site of reaction with lower electron density region
valence bond theory
bonds are created by the overlap of atomic orbitals; bonds localized between adjacent atoms rather than delocalized over several atoms, # of hybrid orbitals formed= # of atomic orbitals combined
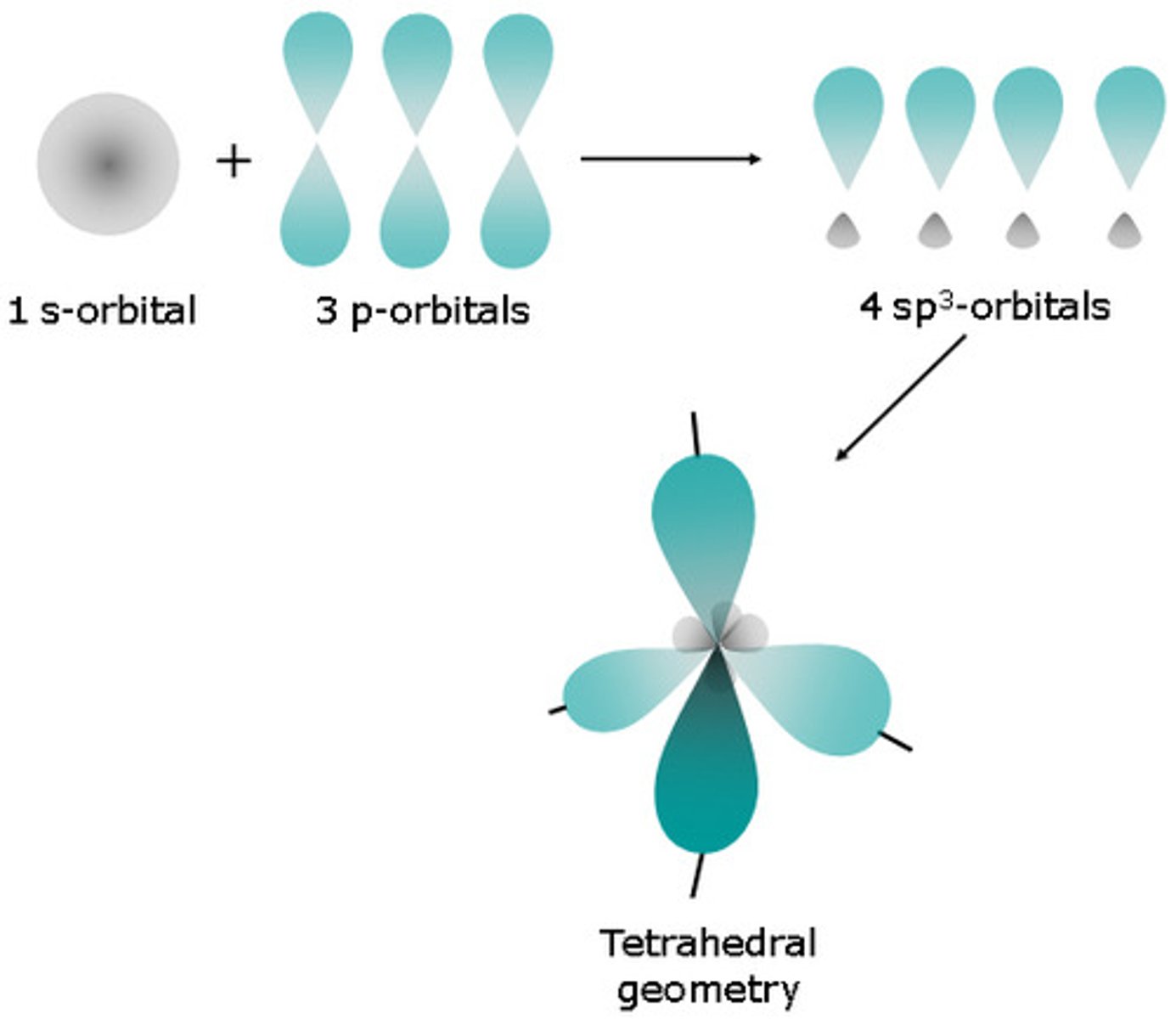
orbital hybridization
1 s + 1 p= 2 sp (2 unhybridized p orbitals)
1 s + 2p = 3sp^2 (1 unhybridized p orbital)
1 s + 3p= 4sp^3 (0 unhybridized p orbitals)
double bond
sp^2 hybridization (sigma + pi bond)
triple bond
sigma bond formed by overlap of the sp hybrid orbitals and one pi bond formed by overlap of parallel 2p orbitals
pi bond
formed by overlap of unhybridized p orbitals on intranuclear axis (side to side); more p in hybrid orbital--> longer and skinnier hybrid--> longer bond length--> less energy to break bond
sigma bond
formed by overlap of orbitals on internuclear axis