Properties of Water- USBT
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
Surface Tension
caused by water molecules being attracted to one another (cohesion)

Cohesion
water molecules sticking together

Adhesion
water molecules sticking to other things

Capillary Action
allows water molecules to move through things (adhesion) like tiny tubes in roots of plants.

Water
is the universal solvent
Specific Heat
the amount of heat needed to raise the temperature of a 1kg sample by 1 degree Celsius
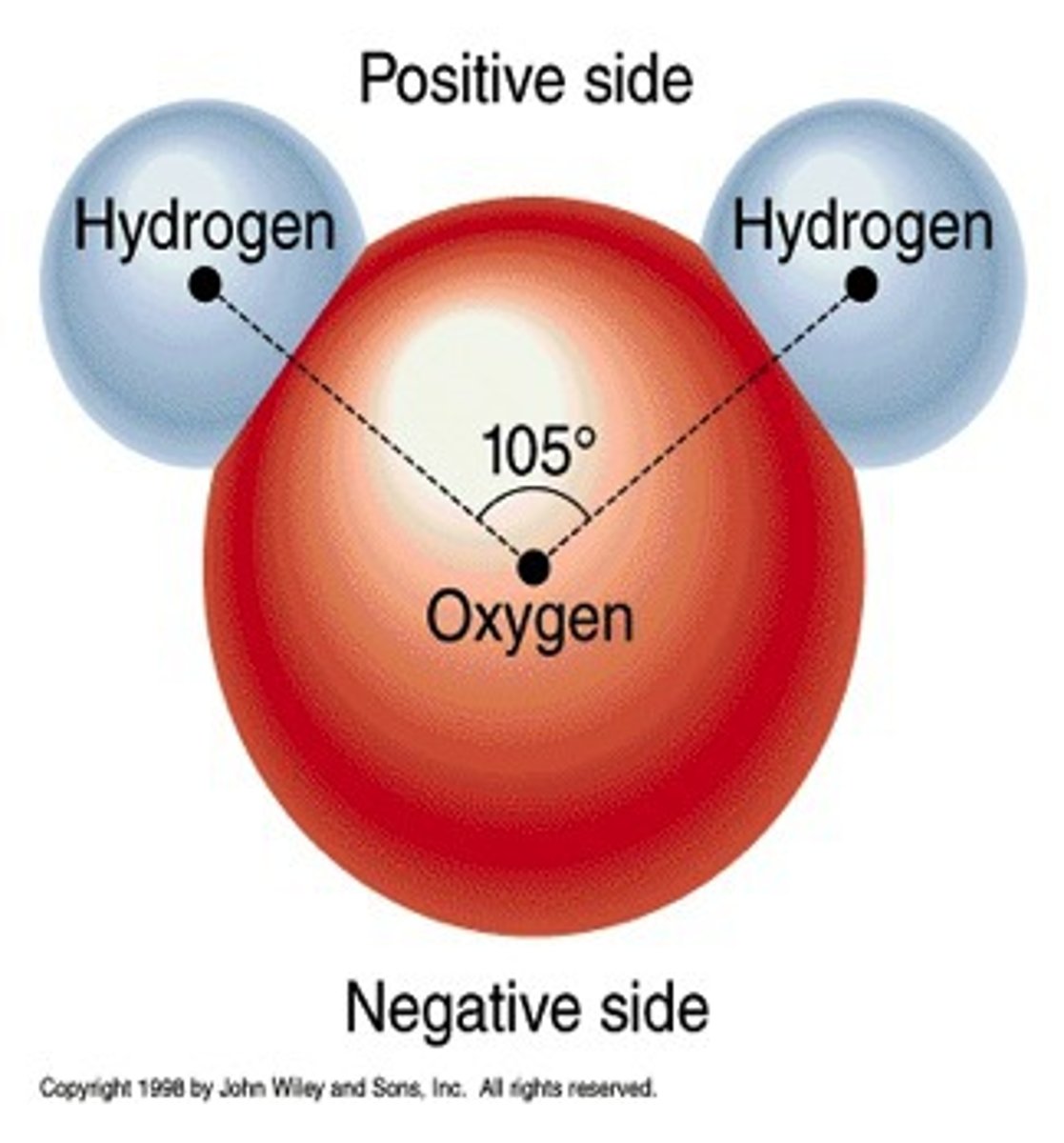
Polarity
one end of the molecule has a positive charge and the other end has a negative charge
Density
The decrease in this is demonstrated in ice compared to liquid, allowing water molecules to expand and float.
Expansion
Water molecules spread apart or space out when water becomes ice, causing ice to float.
Surface Tension
The property of water that allows small organisms like spiders to walk on water.
Universal Solvent
water can dissolve many substances.
Hydrogen bond
The weak bond between water molecules that holds one water molecule to another.