Handout 1B: Synthesis of Amino Acids
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
2 Important Keys of Synthesizing Amino Acids
incorporating the amino and carboxyl groups in the appropriate positions
incorporating the appropriate chiralirty
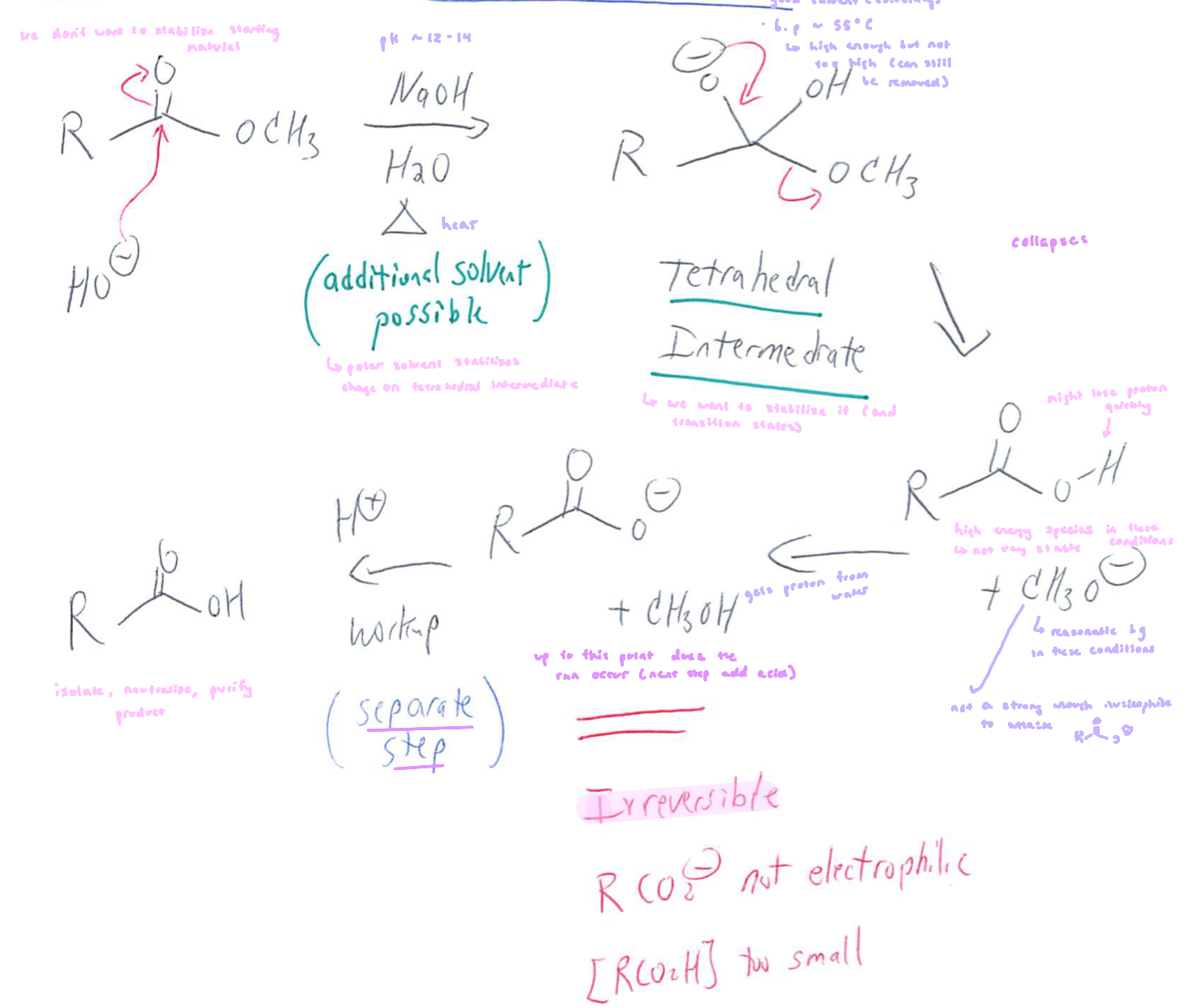
Ester Hydrolysis: Basic
can occur in acidic or basic conditions
saponification (basic): stable species are hydroxide, alkoxide, alcohol and water
any O atom should be neutral of have formal charge of -1
ultimate product is RCO2-, unless there’s an acid workup
not reversible, since the carboxylate is non electrophilic w.r.t alkoxide
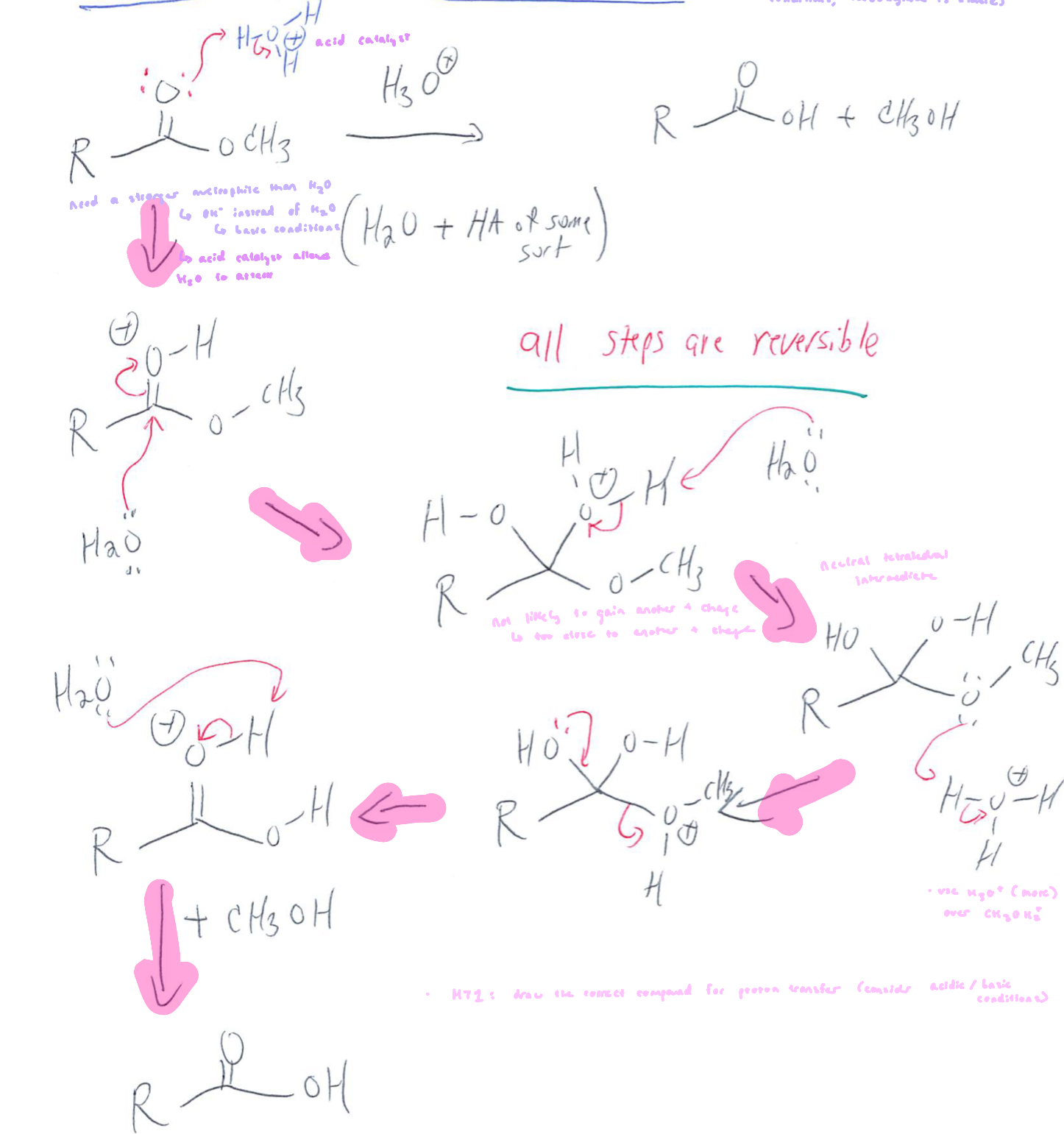
Ester Hydrolysis: Acidic Conditions
reversible
ester must be protonated first to make it electrophilic enough to react w/ water
nucleophile is water
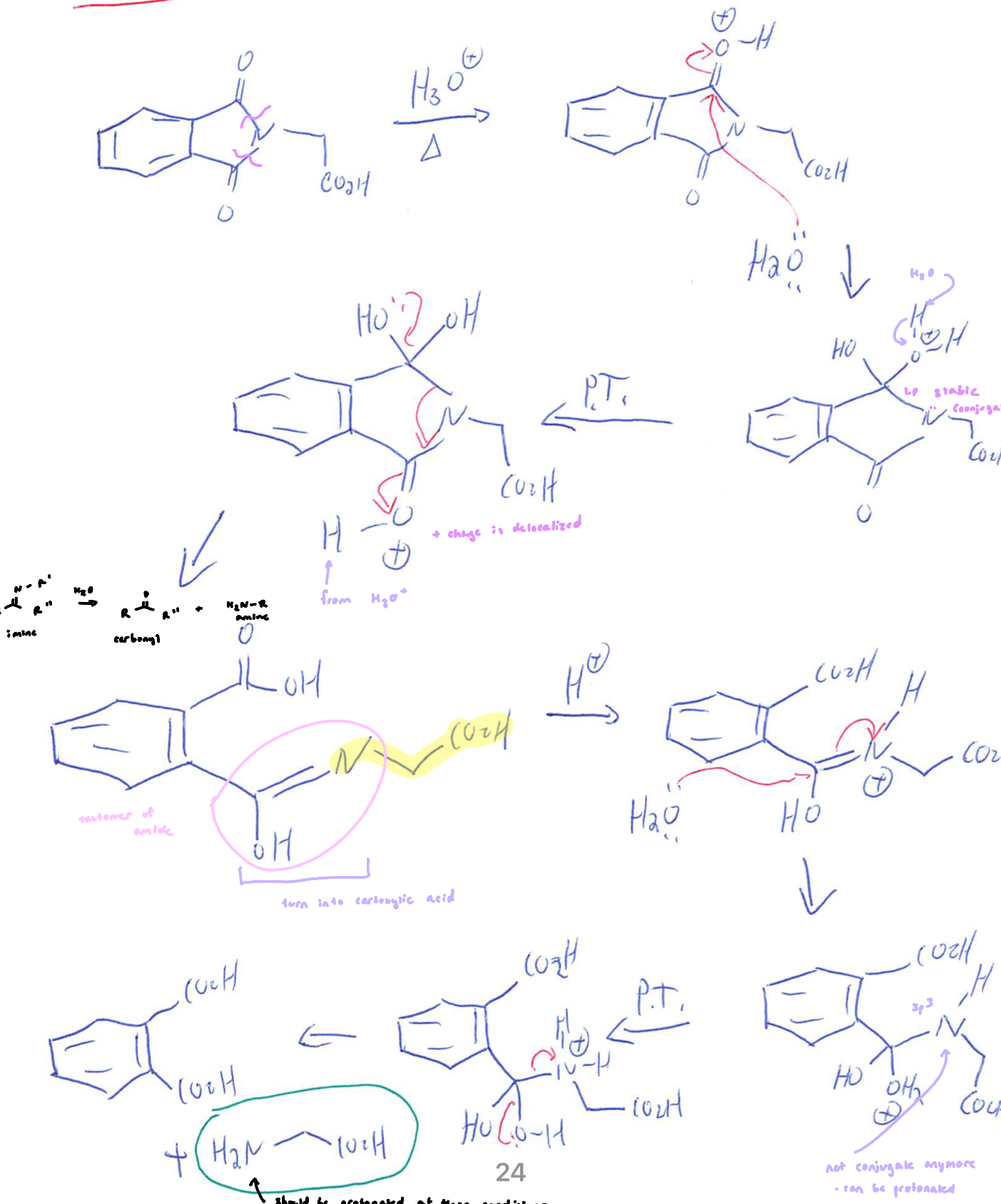
Gabriel Synthesis
involves addition of potassium phthalimide to diethyl-2-bromomalonate (phthalimide allows for a single addition)
the ensuing diester is hydrolyzed to the diacid, then decarboxylated then further hydrolyzed
the imide is hydrolyzed to yield an amine, diacid and phthalic acid
Gabriel synthesis yields a racemic product: the tautomerization following the decarboxylation entails protonation at the ⍺-carbon, which will occur 50:50 from each side
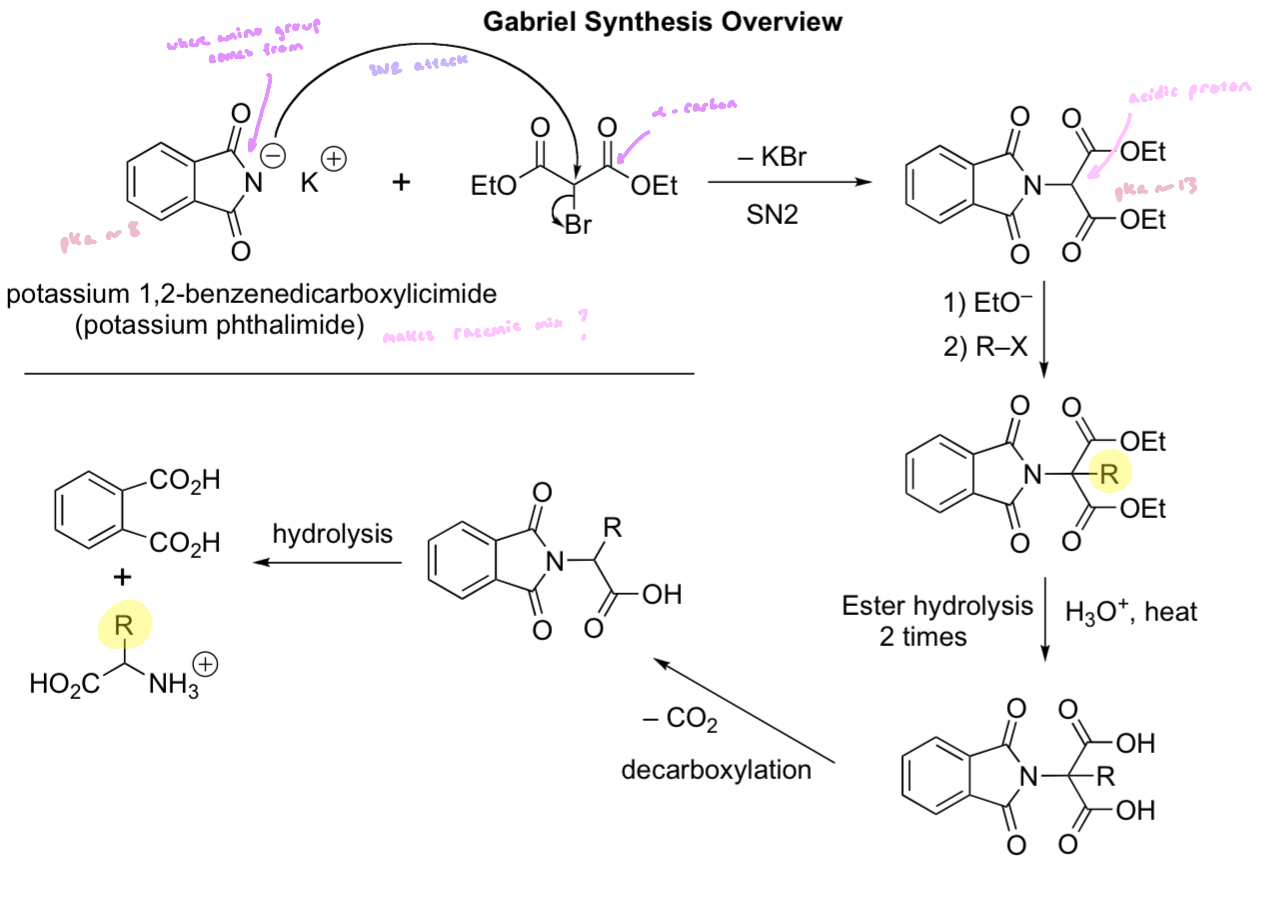
Hydrolysis of Phthalimide
Enantiospecific Process
a starting enantiomer gives predominantly one enantiomeric product; the other starting enantiomer will give the other enantiomeric product
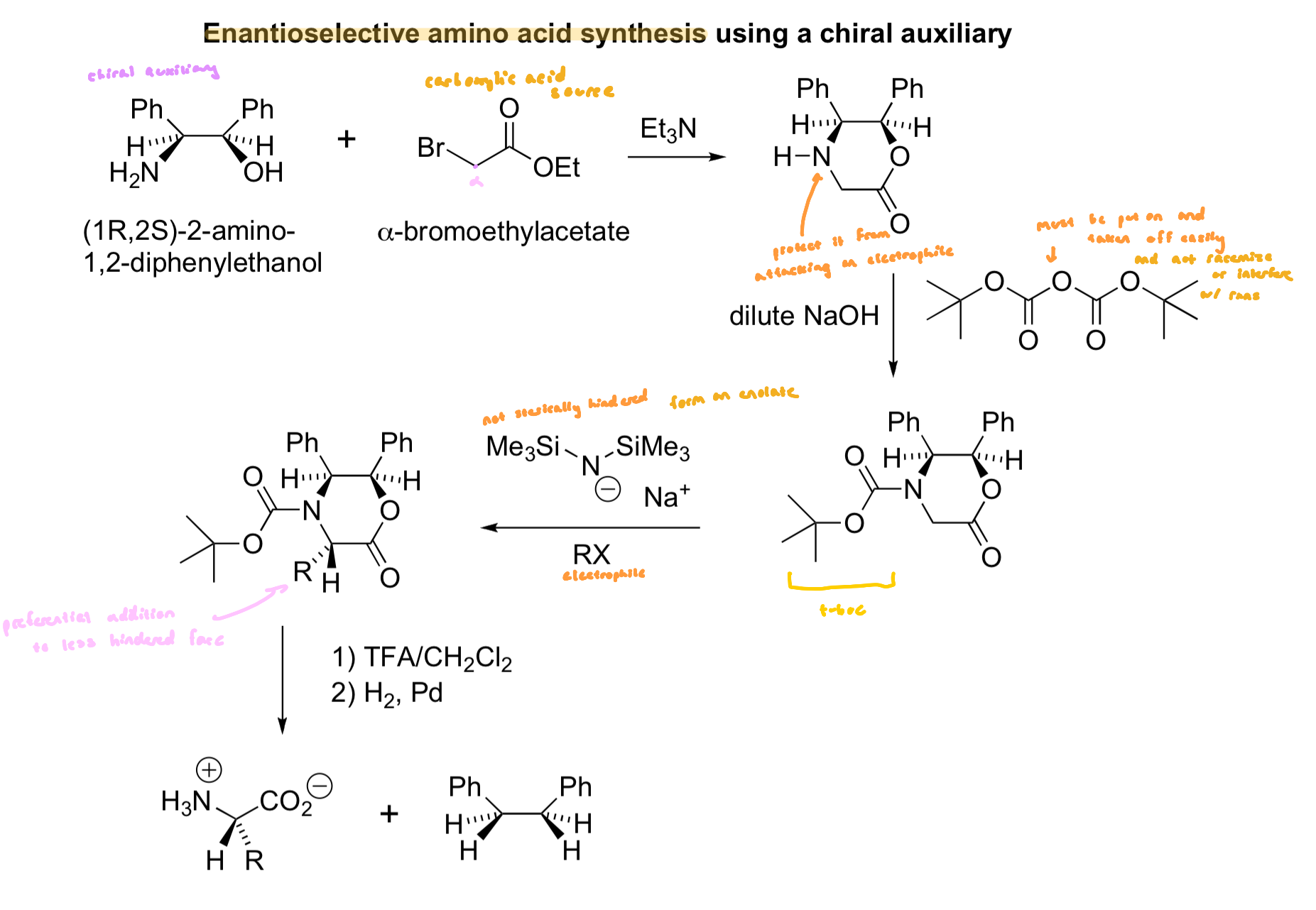
Chiral Auxiliary
homochiral (one enantiomer) helper molecule that is incorporated into an achiral molecule of interest
helps it achieve a stereoselective process and then revmoed, leaving a chiral molecule that is not racemic
we use (1R, 2S)-2-amino-1,2-diphenylethanol for enantiospecific amino acid synthesis
1st step: an SN2 reaction b/w the amine (more nucleophilic than the alcohol) and the ⍺-bromoester yields an intermediate that quickly cyclizes to a six-membered ring
the low nucleophilicity of the alcohol is compensated by the intramolecularity of the reaction
a weak based mops up the acid produced and thus helps convert the remaining starting material from an ammonium to an amine
2nd Step
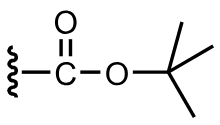
protects the amine as a carbamate (t-Boc)
the carbamate is not nucleophilic
3rd Step
NaHMDS is a strong base that is sterically hinderedl it will pull off any acidic proton, but is a poor nucleophile
the deprotonation (3rd step) is not stereoselective, the less hindered proton may be removed preferentially, but the ensuing anion inverts rapidly
this anion will react from the face opposite that of the large phenyls, which block the electrophile from coming in from the top
this key step is a diastereoselective reaction, and ultimately after completion of all steps, leads to an enantioselective process
Final Steps
TFA removes the Box group and hydrogenolysis of the benzylamine and benzyl ester readily break the benzyl C-N and C-O bonds
Enantioselective A.A Synthesis using a Chiral Auxiliary