Principles of Chemistry
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
Describe metallic bonding in electrostatic attractions
The electrostatic force of attraction between positive metal ions surrounded by a ‘‘sea’’ of delocalised electrons.
Draw a 2D Diagram describing a metal lattice
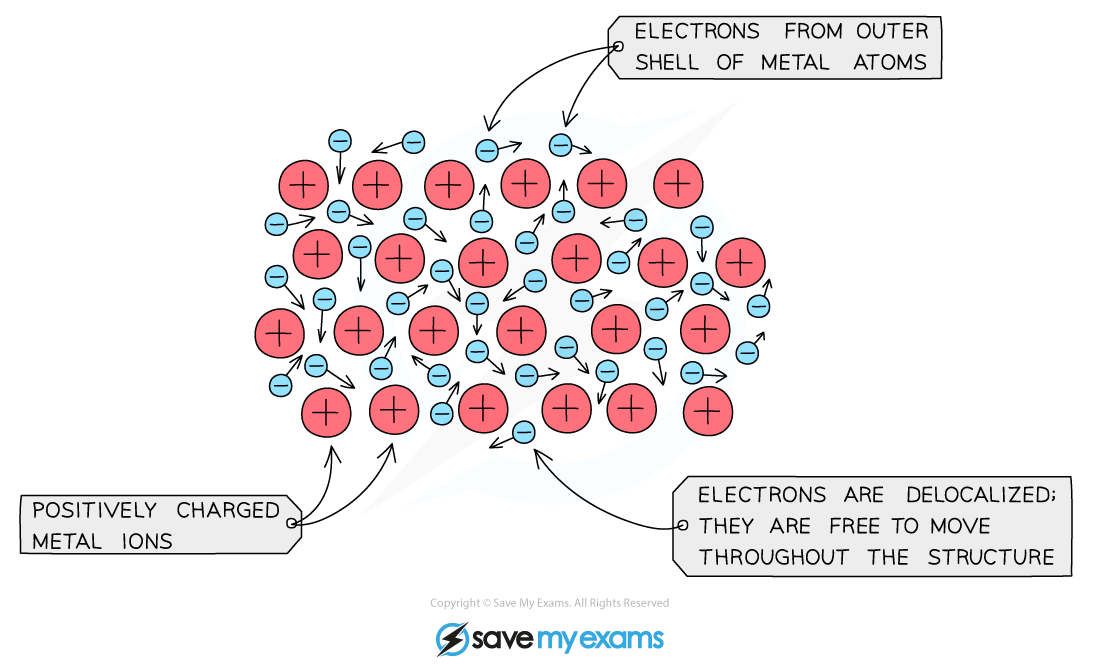
Explain why a metal is ductile/malleable
The atoms/ions are arranged in layers which can slide over each other when a force is applied.
Definition of malleable
This means they can be hammered into shape.
What is electrical current?
The flow of electrons.
Explain the electrical conductivity of a metal
There are delocalised electrons that are able to move and carry the charge through the structure.
How to calculate volume of gas?
Volume of gas = moles of gas x molar volume
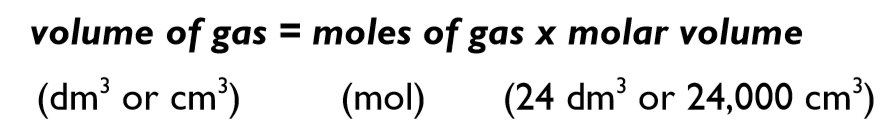
How many moles of gas is there usually?
24,000 cm³ and 24 dm³