Periodic Table- Groups and Periods
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
20 Terms
Group
Elements in the same ____ have very similar chemical and physical properties.
Transition Metals
The name of the middle section of the periodic table.
Noble Gases
Group 18 of the periodic table. These elements are inert (do not undergo chemical reactions under normal conditions).
Periods
Rows of the periodic table
Groups
Columns of the periodic table
Hydrogen, oxygen, helium
Name 3 non metals
Metalloids
Group with properties of metals and non metals. Some are semiconductors - can carry electrical charge
Boron, silicon, germanium
Name 3 metalloids
Metal
The Properties of ___: malleable and ductile, shiny luster, high melting points, good conductors of heat and electricity
Nonmetals
The Properties of ___: not malleable or ductile, low melting points, poor conductors of heat and electricity
Nobel gases, halogens, oxygen family
What families have nonmetals in them?
Hydrogen
What is the lightest element?
Group 1
Alkali Metals
Group 2
Alkali Earth Metals
Group 17
Halogens
Group 18
Noble Gases
Inner Transition Metals
What are the groups highlighted in pink?
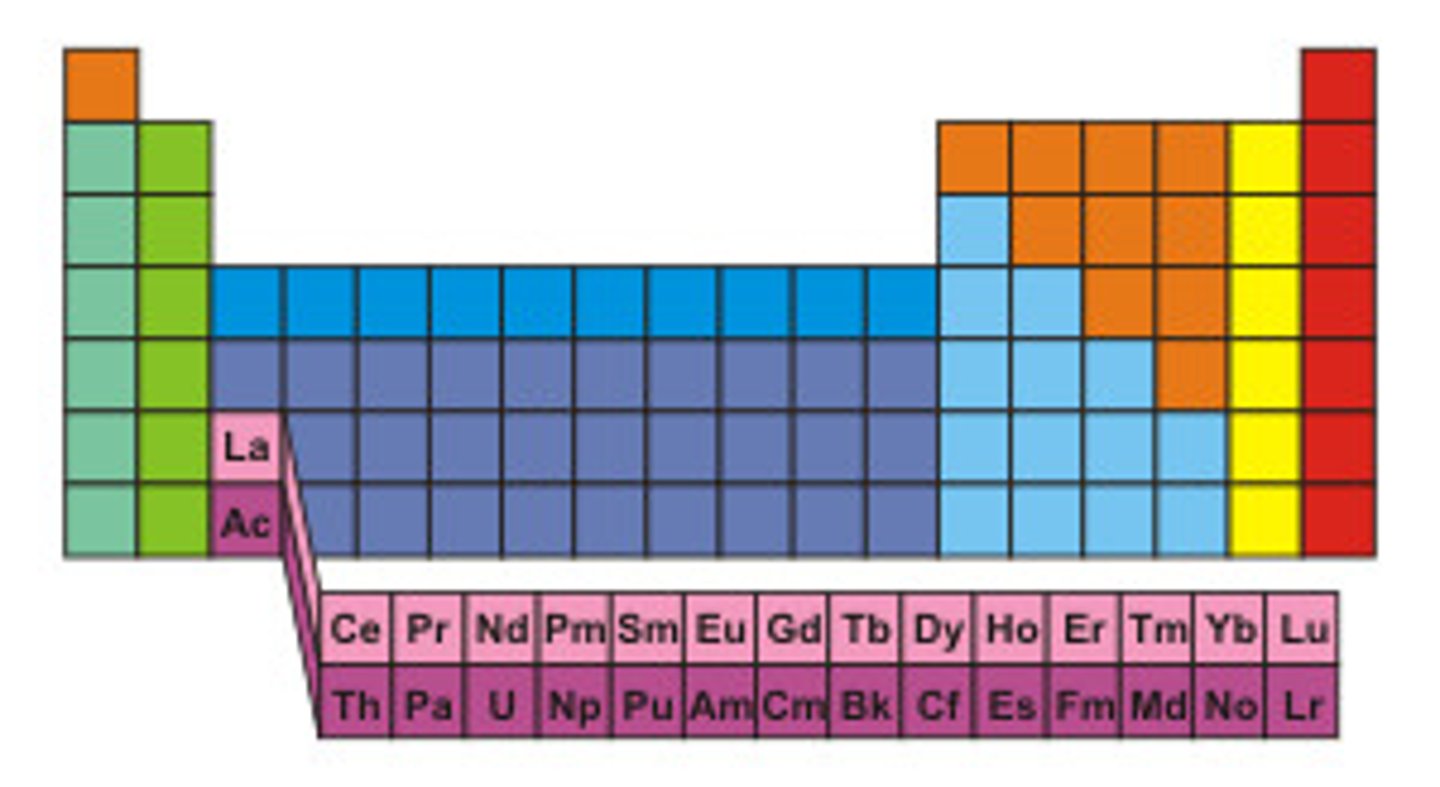
less
Transition metals are ________ reactive than the alkali metals
Groups 1,2,13-18 are called
representative elements
Groups 1 and 17
Which groups on the periodic table are the most reactive?