Chapter 7 - Cellular Respiration and Fermentation
1/64
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
65 Terms
Cellular Respiration
catabolic process which harvests chemical energy from organic compounds
energy releasing process (exergonic)
Aerobic Respiration
uses oxygen in the breakdown of organic molecules to produce energy
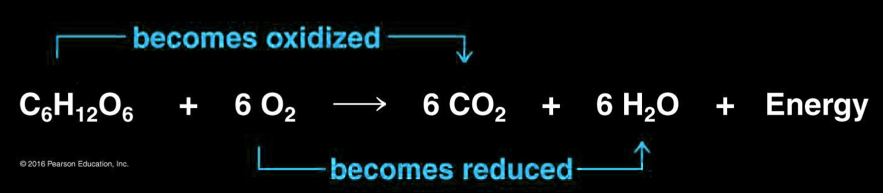
Organic compound + O2 → CO2 + H2O + energy
oxygen draws in electrons due to its high electronegativity
uses a variety of biomolecules like carbohydrates, lipids, proteins
most of the time, glucose is broken down
Anaerobic respiration
uses molecules other than oxygen in the breakdown of organic molecules to produce energy
Principle of redox (what is reducing agent, oxidizing agent, etc.)
during a chemical reaction, electrons are transferred and organic molecules release stored energy
this energy released is used to make ATP
reducing agent becomes oxidized as it loses electrons (becomes more positive)
oxidizing agent becomes reduced as it accepts electrons (becomes more negative)
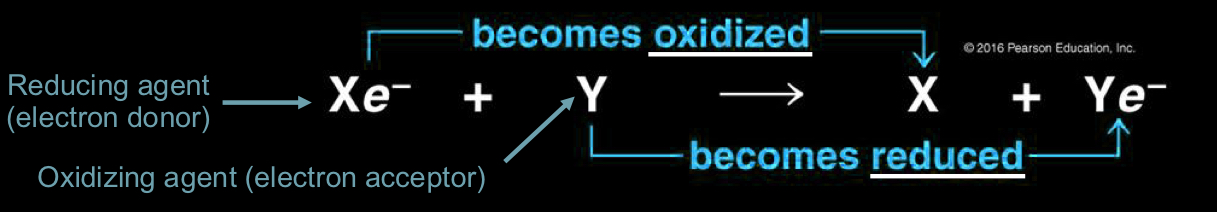
Oxidation in cellular respiration
glucose (C6H12O6) is oxidized and oxygen is reduced
Reducing agent always starts with hydrogen and loses it
Oxidizing agent doesn’t start with hydrogen but gains it
electrons lose potential energy during the transfer
organic molecules are not consumed instantly due to their high activation energy
stepwise energy harvest in cellular respiration
glucose is harvested in a stepwise process
electrons are stripped from glucose step by step
electrons are then transferred along with a proton as a hydrogen atom but are not passed directly onto oxygen
during the intermediate steps, electrons are passed to electron carriers (coenzymes)
NAD+
Nicotinamide Adenine Dinucleotide
main carrier in cellular respiration
oxidizing agent (accepts electrons)
cycles between NAD+ (oxidized) and NADH (reduced)
extremely versatile and is involved in many of the redox reaction steps of cellular respiration
Dehydrogenases
specialized enzymes which catalyze the transfer of 2 electrons and 1 proton to NAD+
a second proton (H+) is also removed which is freefloating in the solution
important in generating ATP due to its accumulation
3 steps of electron transfer in cellular respiration
Electrons are passed from glucose to NAD+ making NADH
NADH transfers the electrons to a series of carriers (in multiple steps)
final electron transfer is to oxygen at the end of the electron transport chain
steps within the ETC release small amounts of energy
oxygen pulls electrons down the chain due to its high electronegativity
3 stages of cellular respiration
Glycolysis
Pyruvate Oxidation and the Citric Acid Cycle
Oxidative Phosphorylation
What is the goal of cellular respiration
to generate ATP released during electron transfers
energy released is used to add a phosphate group to ADP
two methods of phosphorylation
Oxidative phosphorylation
Substrate-level phosphorylation
oxidative phosphorylation
inorganic phosphate is added to ADP to produce ATP at the end of cellular respiration
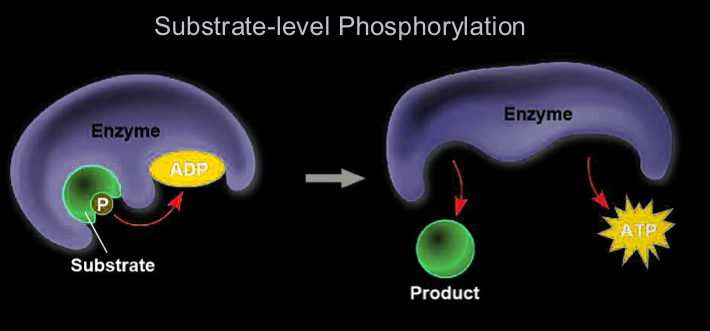
Substrate-level phosphorylation
phosphate group is transferred from a substrate to ADP
occurs during glycolysis and the citric acid cycle
How much potential energy in glucose and ATP?
glucose - 686kcal/mol of potential energy (stored in chemical bonds)
ATP - 7.3kcal/mol of potential energy
how many moles of ATP does cellular respiration create and how much energy is that?
32 molecules of ATP x 7.3 = 233.6kcal/mol
Glycolysis
“sugar splitting”
core process used by all cells
occurs in the cytosol unlike cellular respiration (in eukaryotes)
may occur with or without oxygen
splits a single 6C glucose into two 3C pyruvate molecules
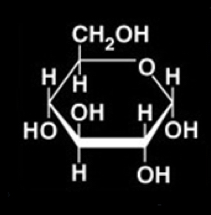
Glucose structure (draw it)
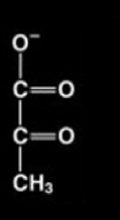
pyruvate structure (draw it)
Energy investment phase of Glycolysis
use ATP to create phosphorylated intermediate
Alteration of carbon bonds to turn phosphorylated intermediate into fructose
fructose gets phosphorylated using energy (transform ATP into ADP)
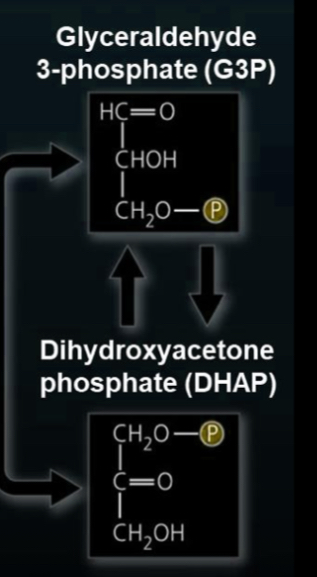
6C sugar is split into two 3C sugars (G3P and DHAP)
Isomerase turns DHAP into G3P, so now there’s two G3P molecules
structures of G3P and DHAP (draw them)
Energy Payoff Phase of Glycolysis
two steps
a) G3P is oxidized by transferring electron from NADH to NAD+ where energy is harvested
b) energy harvested is used to add a phosphate group to the oxidized G3P to create a high energy molecule
phosphate group is transferred to 2 ADP molecules through exergonic reactions to create 2 ATP molecules
enzyme moves phosphate groups to create high energy molecules
double bond is created through the removal of water which increases energy of substrate molecules
phosphate group is transferred to 2 ADP which creates 2 molecules of pyruvate and ATP
net reactions of glycolysis
Glycolysis → 2 pyruvate + 2 H2O
4 ATP formed - 2 ATP used → 2 ATP
2 NAD+ + 4 e- + 4 H+ → 2 NADH + 2H+
how efficient is cellular respiration in terms of extracting energy from glucose
less efficient since only 1/3 of energy is left
What component is the oxidizing agent in the basic cellular respiration equation?
Oxygen (gets reduced as it accepts H+)
How many electron(s) and hydrogen atom(s) are transfered to NAD+ from a redox reaction
2 electrons go to every molecule and hydrogen becomes free floating in the cytoplasm
during steps 1 and 3 of glycolysis, what is happening
phosphorylating glucose intermediate
(phosphate comes from ATP)
what is produced during steps 7 and 10 of glycolysis
ATP is produced by phosphorylating ADP
fermentation
allows continuous production of ATP by substrate-level phosphorylation of glucose
requires the transformation of NADH to NAD+ for the cycle to continue
happens through the reduction of pyruvate or its derivatives
occurs in a cell when no oxygen is available
cons of fermentation
Since it occurs in a cell when no oxygen is available:
far less efficient as less ATP is returned
but is the only efficient way of producing energy for some cells in extreme conditions
anoxic
lack of oxygen
what determines whether a cell will use aerobic respiration or fermentation
presence or absence of oxygen
how many molecules of ATP produced in aerobic respiration vs. fermentation
32 ATP molecules in aerobic respiration
2 ATP molecules in fermentation
2 types of fermentation
Alcohol fermentation
lactic acid fermentation
What do bacteria/fungi and muscle cells use fermentation for
bacteria/fungi - dairy production
muscle cells - carried to liver and converted back into pyruvate
where does pyruvate oxidation take place
mitochondria (for eukaryotes)
cytosol (for prokaryotes)
what happens after glycolysis
pyruvate (in the presence of oxygen) enters either mitochondria (for eukaryotes) or stays in cytosol (in prokaryotes)
oxygen is used to harvest the ¾ energy (1/4 harvested from glycolysis) that is left in pyruvate
pyruvate oxidation
involves multiple enzymatic steps to oxidize pyruvate into acetyl-CoA
occurs in the matrix of the mitochondria
NAD+ is reduced to NADH
acetyl-CoA then continues into the citric acid cycle
steps of pyruvate oxidation
1 carbon is removed from pyruvate to create CO2
leftover molecules gives up energy for NAD+ to be reduced into NADH
this stores energy which is used later in ATP production
CoA is added to replace the removed carbons spot
creates Acetyl CoA
Products of Citric Acid Cycle
3 NADH
2 CO2
3 H+
1 ATP
1 FADH2
for every turn of the cycle
how many turns of the citric acid cycle produces 1 glucose molecule
2 turns
steps of the citric acid cycle
Acetyl CoA enters and transfers its two-carbon group onto oxaloacetate and then CoA leaves
intermediate molecule rearranges
intermediate is oxidized as it releases one carbon as CO2, NAD+ is reduced to capture energy
second carbon is released as CO2 and another NAD+ is reduced into NADH with a freefloat H+
substrate-level phoshorylation creates a small amount of ATP
electrons are transferred from the substrate to FAD to form FADH2 for later ATP production
Last NADH is produced with a freefloat H+ and original 4 carbon molecule (oxaloacetate) is regenerated to restart the cycle
Flavin Adenine Dinucleaotide (FAD)
an electron carrier similar to NAD+ that gets reduced to FADH2
FAD binds to 2 H+ and 2 electrons to form FADH2
How much ATP is produced during fermentation and where does it come from?
only 2 ATP molecules from every cycle
comes from glycolysis
where in the cell does pyruvate oxidation/citric acid cycle occur? How does this differ between eukaryotes and prokaryotes?
occurs in mitochondrial matrix in eukaryotes
cytosol in prokaryotes
what is the initial molecule of the citric acid cycle?
oxaloacetate
what are the products of pyruvate oxidation
NADH and Acetyl CoA and CO2 per molecule of pyruvate
(2 of each per molecule of glucose)
how much energy is stored, and in what form(s), during each turn of the citric acid cycle
3 molecules of NADH per turn
2 moles of FADH2 per turn
Oxidative Phosphorylation - where its energy is stored, what the process requires, and two processes involved
most of the energy from glucose remains stored in NADH and FADH2
this process requires enzymes embedded in the inner membrane of mitochondrion in eukaryotes or cell membrane of prokaryotes
involves two processes
Electron Transport Chain
Chemiosmosis
Electron Transport Chain (ETC)
Series of redox reactions through membrane-bound electron carriers
has several multi-protein complexes
what are the complex proteins of ETC
prosthetic groups which are required for catalysis
many contain iron to bind electrons
ETC Proteins
FMN (flavin mononucleotide)
first protein of the chain
FeS (iron sulfur protein)
found in complexes 1, 2, and 3
Cyt (cytochrome)
contains heme (iron)
Steps of ETC
Complex 1
NADH becomes oxidized into NAD+ as it releases 2 electrons that are passed onto FMN in complex 1
FMN passes electrons onto FeS
FeS passes electrons to Q (shuttle molecule)
pumps out hydrogen into inter membrane space, and returns NAD+ to the matrix
Complex 2
FADH2 interacts with complex 2 and becomes oxidized, passing its electrons onto FeS
FeS passes electrons to Q (shuttle molecule)
NOT a proton pump
Ubiquinone (Q)
moves electrons received from Complexes 1 and 2 and moves them to Cyt b in complex 3
Complex 3
Cyt b passes electrons to FeS to Cyt c1
pumps out hydrogen into intermembrane space
Cyt C (shuttle protein)
moves electrons received from complex 3 and moves them to Cyt a in complex 4
Complex 4
cyt a passes electrons to cyt a3
cyt a 3 moves electrons out of complex 4
electrons combine with oxygen (last electron acceptor) and H+ to form H2O and more H+ are pumped
continuous pumping of H+ creates the proton-motive force (electrochemical gradient between matrix and inter membrane space)
Shuttle molecules
small hydrophobic molecules that move electrons from complex to complex
not proetins
not firmly in place on the membrane which allows them to move around
how does proton pumping work
transfer of electrons releases energy which is used to pump H+ into the intermembrane space membrane space
complexes 1, 3, and 4 are proton pumps, but not complex 2
NADH moves 3 H+ for every 2 H+ from FADH2
Proton-motive force
electrochemical gradient produced by the ETC
used to drive ATP production through membrane-bound ATP synthase
ATP synthase moves hydrogen down gradient which phosphorylates ADP into ATP
ATP synthase
synthesizes ATP from ADP and inorganic phosphate using the H+ gradient
protons bind to rotor causing it to spin and phosphorylation happens
spinning of the rotor powers the production of ATP through catalytic site on the knob
Chemiosmosis
Process where a proton gradient across a membrane is used to drive cellular work to produce ATP
works as an energy-coupling system
energy is created through the gradient from redox reactions
overlaps and connects to the ETC, but they aren’t the same, they just happen at the same time
needs the ETC chain in order to work (since ETC creates the H+ gradient)
ATP synthesis occurs here
what steps of cellular respiration require oxygen and what steps do not
Glycolysis - doesnt need oxygen
pyruvate oxidation - needs oxygen
citric acid cycle - requires oxygen
Oxidative phosphorylation (ETC and chemiosmosis) - requires oxygen
which step of cellular respiration is a true cycle
Citric Acid Cycle (AKA Krebb’s Cycle)
since the initial molecule, oxaloacatate, is regenerated at the end of the cycle
ETC in anaerobic respiration
final electron acceptor may be sulfate, nitrate, sulfur, or fumarate
final electron chain is less electronegative than oxygen so less energy is produced overall
generates more ATP than fermentation but less than aerobic respiration
facultative anaerobes
can survive using fermentation/anaerobic respiration OR aerobic respiration
ex. listeria monocytogenes
obligate anaerobes
can ONLY use fermentation or anaerobic respiration and cannot survive in oxygen
oxygen is toxic to them since they’ve become adapted to survive without it
Ex. clostridium botulinum
How much ATP is produced in cellular respiration
1 NADH = 2.5 ATP
1 FADH2 = 1.5 ATP
32 molecules of ATP
what other energy resources besides glucose are used in cellular respiration and how
carbohydrates - large polysaccharides can be broken down into monomer sugars
proteins - digested into amino acids and then deaminated before entering glycolysis or converts directly into Acetyl CoA
fats - digested to glycerol and fatty acids
Glycerol becomes G3P
Fatty acids go through beta oxidation to produce Acetyl CoA and NADH/FADH2