principles of chemistry
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
23 Terms
Metabolism
The whole sum of reactions that occur throughout the body that provide the body with energy
Vital force theory (vitalism)
first stated by Jacob Berzelius in 1809
belief that living organisms possess usique properties not explained by ordinary processes
that organic material can only come from organic things
this the vital force
3 ways vitalism was debunked
Wholer’s synthesis f Urea (1828)
Pastuer disproving spontaneous generation
Buchner’s Cell-free fermentation
Wohler (1828)
Inorganic chemist
Discovered urea while mainly interested in synthesizing ammonium cyanate
Pasteur (1859)
Microbiologist
Was set to disprove spontaneous generation
Buchner
Zymologist (studies fermentation)
Yeast extract with no living yeast fungi can form alcohol from sugar solution
Discovered zymase from yeast that caused glycolysis
Does plant metabolism behave on the basic principals of chemistry and physics that govern inanimate matter
yes
What determines the elemental identity of an aton
proton
electronegativity
ability of an atom to attract electrons towards itself
what is a polar molecule
have a slightly positive and negative end
Hydrogen bond
when 2 molecules come close to each other and their charges slightly attract each other
why is water the universal solvent
IT can surround and break apart ions and molecules because of water’s polarity and hydrogen bonding
What contributes to the earth’s temperature
high specific heat capacity of water
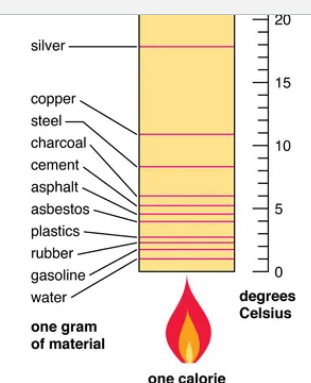
what is the specific heat capacity

Benefits of having a high specific heat capacity

What are the effects of having a high heat of vaporization
It makes it so that a lot of energy is required to change something from liquid to gas
Why does water have a high specific heat capacity and high heat of vaporization

What is a weird property of frozen water
Water molecules in ice arranges itself in a hexagonal structure
Makes ice less dense than water
Surface tension
Result of cohesive forces amongst water molecules
gives water resistance to outside pressures
pH scale
measures the acidity or alkalinity of a solution
0-14, alkalinity - acidity
What does pH measure
The concentration of hydrogen ions in a solution
What does pH stand for
Potential of hydrogen