L16: Fermentation and syntrophy
1/15
Earn XP
Description and Tags
A set of flashcards covering key concepts and details from the lecture on fermentation and syntrophy.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
16 Terms
Fermentation occurs in the absence of?
Suitable electron acceptors such as O2, NO3 , or Fe3+
What are the main problems faced by fermentative bacteria?
Maintaining REDOX balance i.e. typically electrons derived from oxidiation of the growth substrate would be donated to the EA e.g. Fe3+ → Fe2+
Obtaining sufficient ATP without an electron transport chain.
What is one way bacteria reduce part of the growth substrate during fermentation?
By excreting products like lactate, H2, acetate, or ethanol
i.e. NAD+ regenerated from oxidation of NADH in pyruvate → lactate
This leads to dissipation of reducing power, without this the cell would run out of NAD+ and be unable to continue fermentation.
What is the process of substrate level phosphorylation in fermentation?
ATP is generated by the transfer of a phosphate group from a high-energy substrate to ADP.
What does a balanced fermentation require?
A reduction for each oxidation, and it can be calculated to ensure know all fermentation products.
Example of Fermentation Balance

If 1.9 mol of ethanol and 1.9 mol CO2 are produced from 1 mol of
glucose, is this a balanced reaction?
Should be 2 moles of each, i.e., some carbon is missing
Other fermentation products are formed, could be a contaminant.
What are the two main products of ethanol fermentation?
Ethanol (C2H5OH) and carbon dioxide (CO2).
How do bacteria maintain redox balance during fermentation?
By reducing part of the substrates like in formation of lactate and producing compounds like H2 (way of dissipating reducing power using H+ derived from H2O)
Why do many bacteria produce acetate while fermenting?
Allows production of ATP through SLP
Electrons obtained from oxidation of pyruvate are donated to ferredoxin and subsequently to H2O to generate Hydrogen
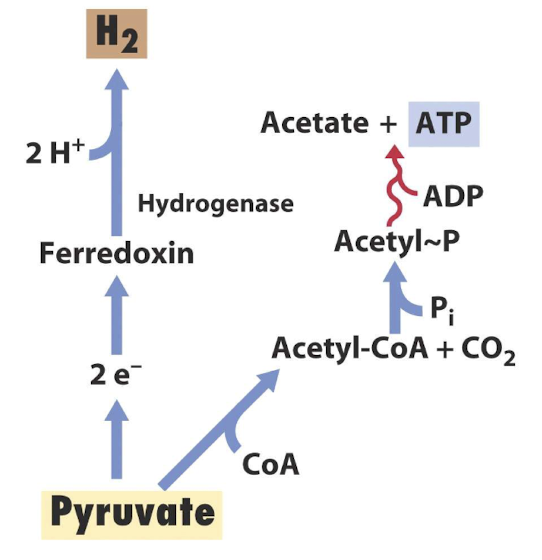
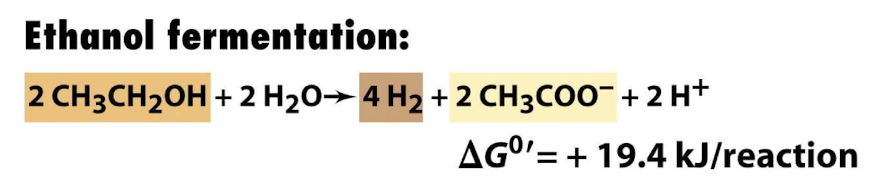
Why is ethanol fermentation not energetically favourable?
It has a positive change in standard free energy (+ 19.4 kJ/ reaction) meaning it requires energy input to proceed and is not spontaneous under standard conditions, bacteria have not been shown to do this in pure cultures
What allows ethanol fermentation to proceed in cooperation with methanogens?
Methanogens consume the H2 produced by combining with CO2 by methanogenesis, much more energetically favourable
The coupled reaction of ethanol fermentation and methanogenesis by TWO prokaryotic species is exergonic
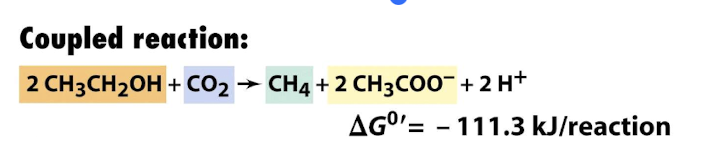
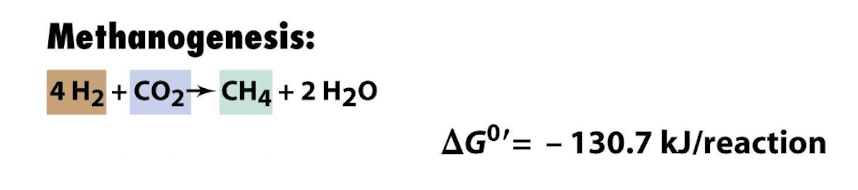
standard free energy change defined under_____
Under 1 atm pressure, at pH 7 and 1M concentrations
How is the reaction driven forward?
Even though the standard free energy change is positive, cells don’t run reactions at “standard conditions.” They run them at the actual intracellular/extracellular concentrations and pressures, where
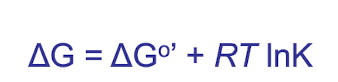
thus if the equilibrium constant K (influenced by conc of products becomes smaller than 1, then lnK becomes negative
How do bacteria achieve lowered [H2] in nature?
They typically do it via syntrophy: a partner organism (often a methanogen or sulfate reducer) rapidly consumes H₂ (and sometimes acetate), keeping extremely [H2] low.
Under those conditions, the ethanol oxidation/fermentation becomes thermodynamically favourable
What is interspecies hydrogen transfer?
Where energetically unfavourable rxns like ethanol fermentation by a fermentor works as the other organism (a methanogen) keeps the concentration of H very low via oxidation
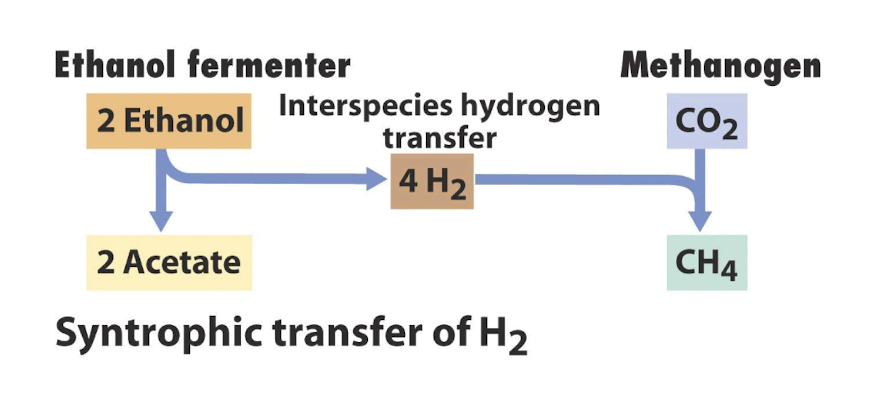
What is syntrophy in the context of microbial fermentation?
Syntrophy is a cooperative interaction between different microbial species where one species' metabolic byproducts serve as nutrients or electron acceptors for another species, often enhancing fermentation efficiency.