IB Chemistry HL topics 1-21 + option B
1/672
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
673 Terms
Alkane combustion reaction
Complete : results in CO2 forming
Incomplete results in CO or C forming
What causes free radical substitution of alkanes
UV radiation
Define homolytic fission
Homolytic fission is a type of chemical bond cleavage in which a covalent bond breaks, and each atom retains one of the shared electrons. This results in the formation of two free radicals, each with an unpaired electron.
Define free radical
A free radical is an atom, molecule, or ion that contains an unpaired electron. It is highly reactive as they are unstable.
Free radical substitution steps
Initiation: photochemical homolytic fission of the bond between two halogen atoms due to incident UV radiation.
Propogation: Free radical + Molecule
Termination: Free radical + Free radical
Why are alkenes reactive
Pi bond is weaker than sigma bond
double bond is electron dense
Alkene addition reaction types
Halogenation
Hydrohalogenation
Hydrogenation
Hydration
Polymerisation
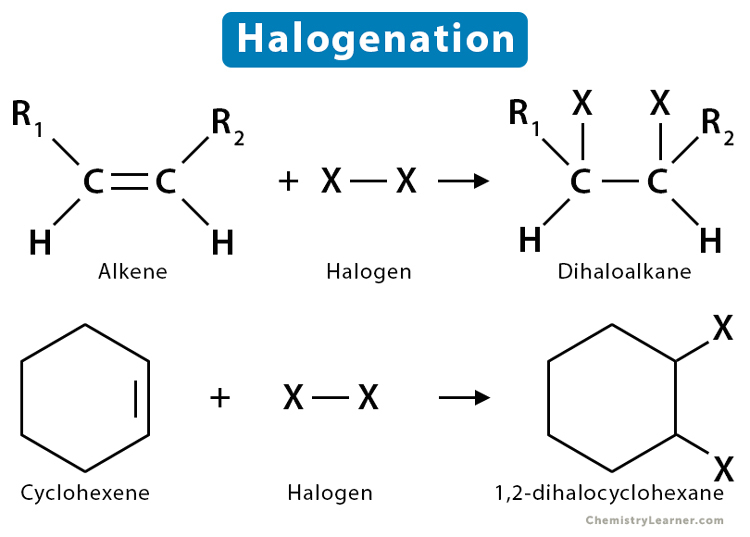
Halogenation reaction + condition
no condition
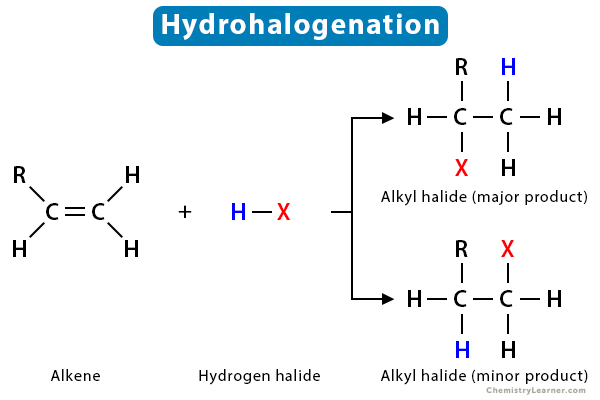
Hydrohalogenation reaction + condition
Heat
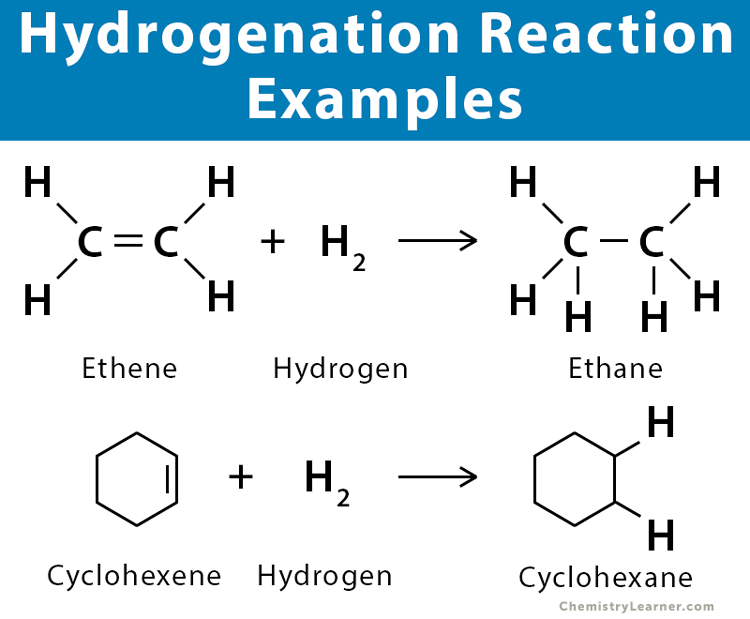
Hydrogenation reaction + condition
150°C + Ni
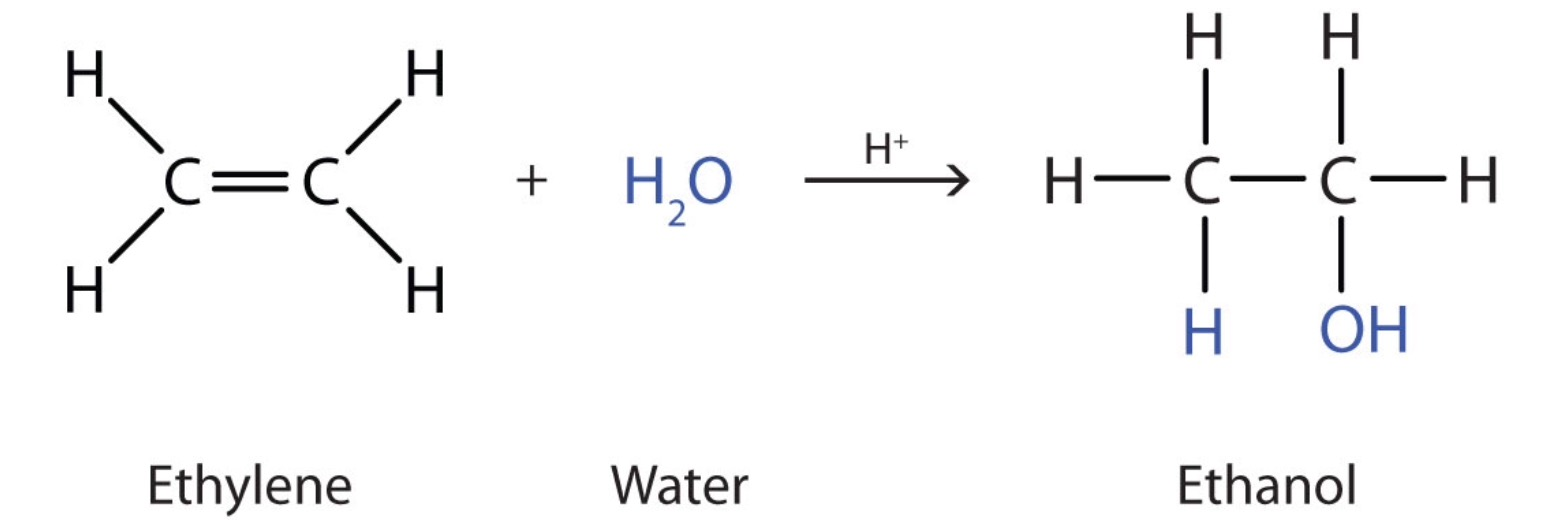
Hydration reaction + condition
Concentrated sulfuric acid + heat
Polymerisation reaction + condition
High temp + pressure + catalyst
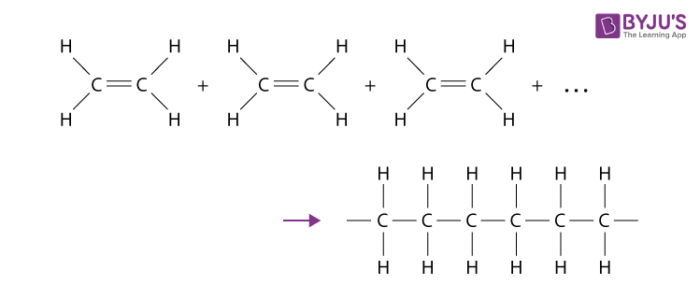
Combustion of alcohols…
completely and selectively oxidises the carbon atom attached to the -OH group
Alcohol oxidation catalyst and colour change
KMnO4 / H+ (aq) + heat
Purple → Clear
Primary alcohol oxidation reaction
Primary Alcohol → Aldehyde → Carboxylic acid
note: You don’t need to know the intermediate step!
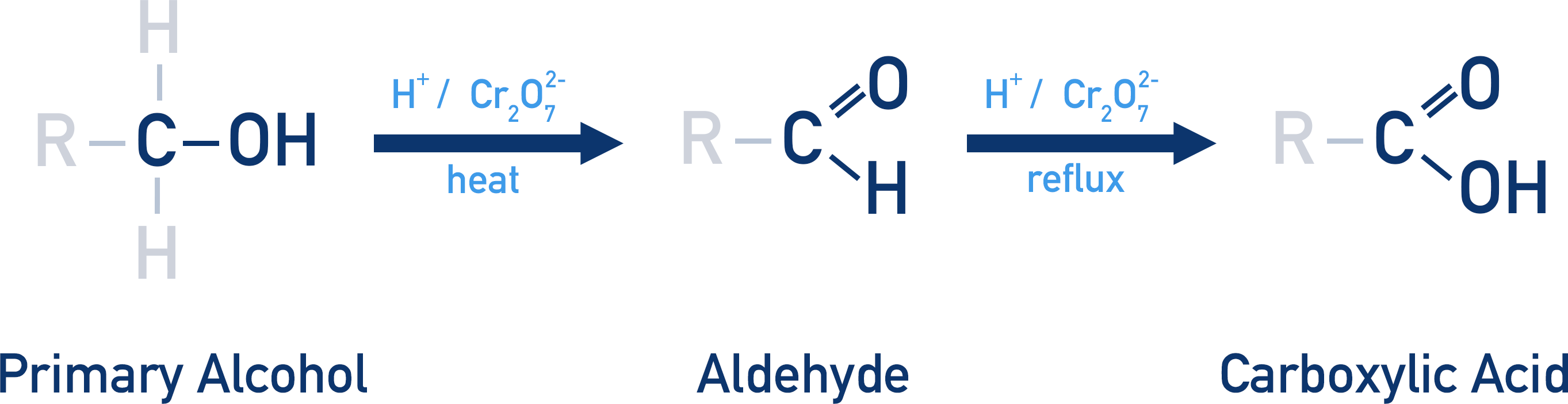
Secondary alcohol oxidation
Alcohol → Ketone
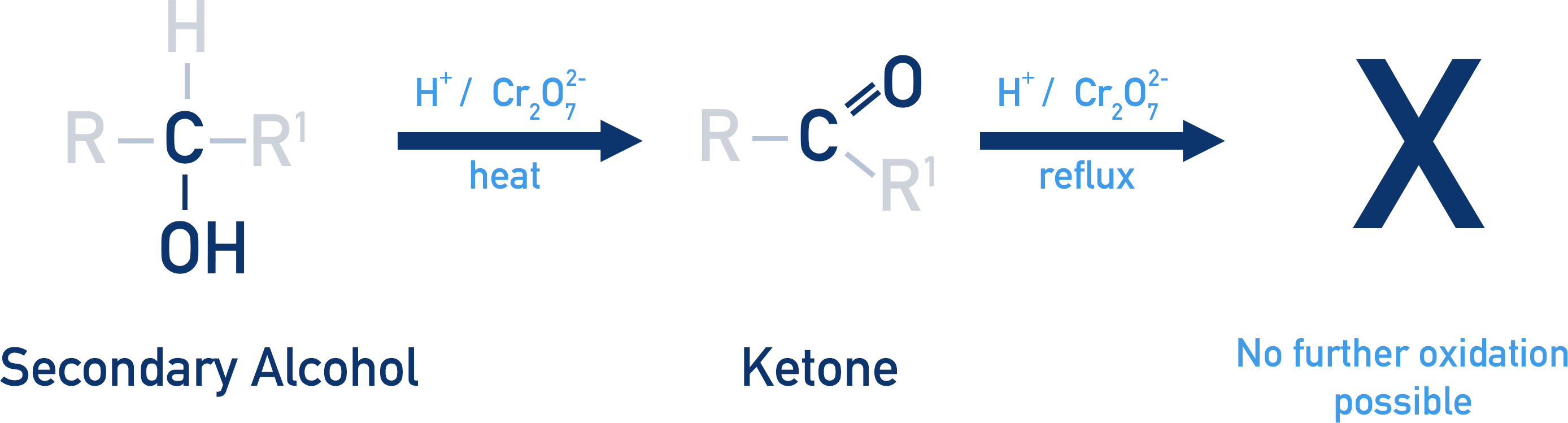
Tertiary alcohol oxidation
resistant to oxidation
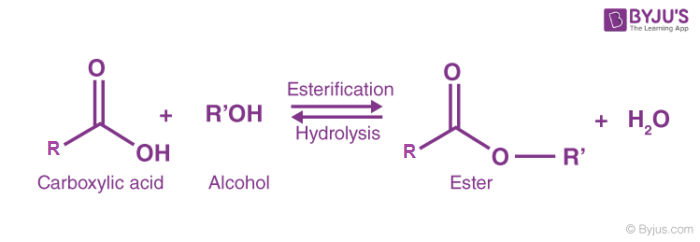
Esterification reaction + catalysts
Alcohols react with carboxylic acid to form esters in a condensation reaction.
Condition: Heat + conc. H2SO4
conditions for sigma bonds
two S-orbitals
one S-orbital and P-orbitals
Two P- orbitals in the same axis
Pi bonds
not as strong as sigma bonds
overlap of Py and Pz orbitals lengthways
occurs when two atoms come close to each other
double / triple bonds are electron dense
Define stereoisomers
A compound with the same structural formula, but arranged differently in space
Define cis-trans isomer
When two compounds have the same structural formula, but groups are arranged differently around a double bond or ring
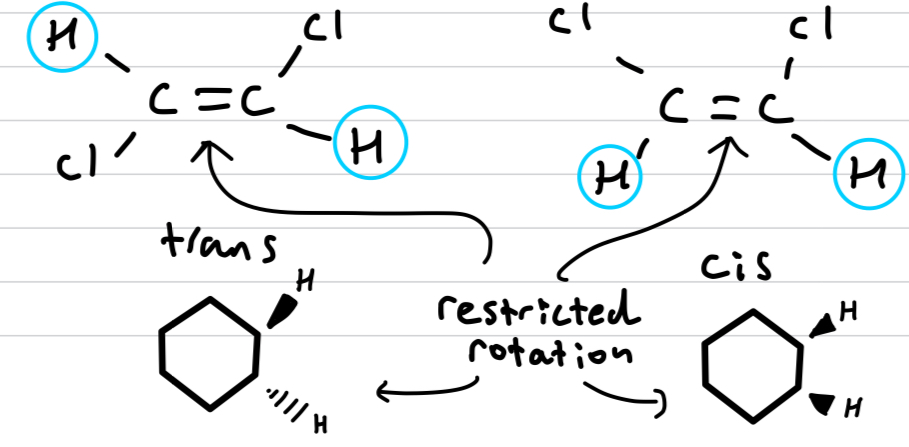
Why are cis-trans isomers configurational and not conformational?
The double bond or ring restricts the rotation
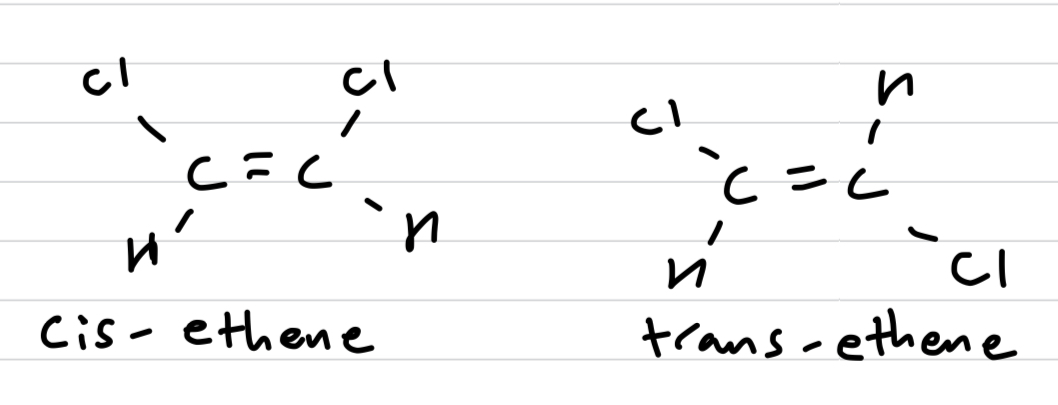
Which cis-trans isomer has a higher boiling point and why?
Cis-ethene, because of the assymetrical distribution of charge forming dipoles, meaning it has both LDF and dipole-dipole intermolecular forces.
Trans-ethene on the other hand is symmetrical in distribution of charge and hence is a non-polar molecule, and only has weaker LDF.
How do you determine priority in naming cis-trans isomers
higher molecular mass = higher priority
E/Z naming system
Cis = Z
Trans = E
What is needed to exhibit optical isomerism
There must be four different groups attached to a Carbon ‘centre’
What type of reaction is esterification
Condensation
Define Chiral
non-superimposable mirror image (assymetric)

Define enantiomer
one of a pair of optical isomers, which are mirror images of each other
Define racemic mixture
an equimolar mixture of two enantiomers (mirror pair) of chiral compounds
Plane polarised light
light that vibrates in one plane only, radiation can be polarised at different rotations depending on which enantiomer it passed through in the pair
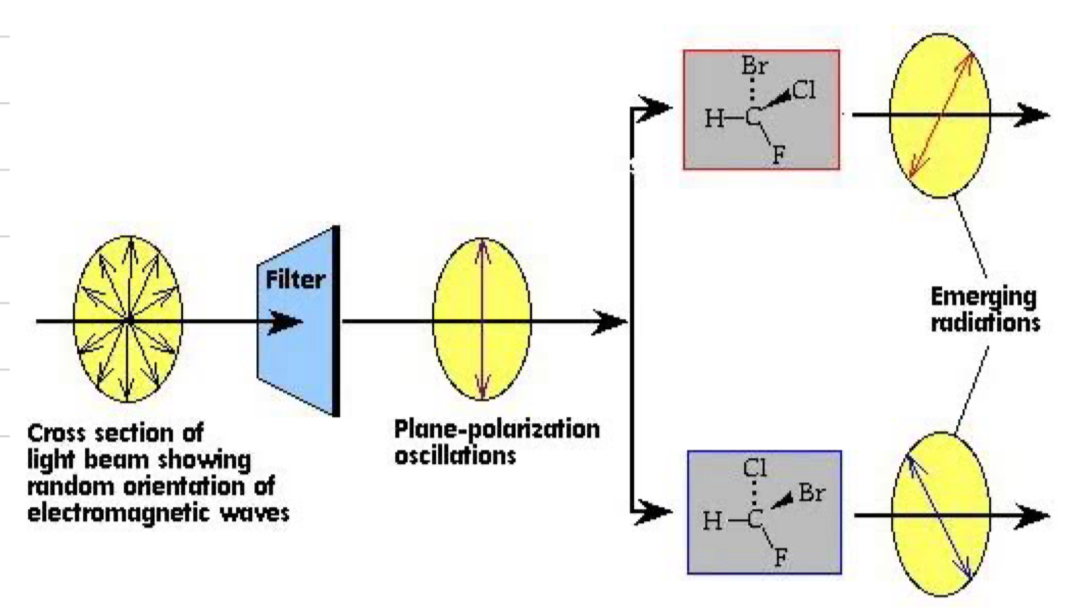
Define optically active
optically active means the compound is capable of polarising the plane of light
Physical and chemical properties of enantiomers
Physical: identical except rotation of plane polarisation
Chemical: Identical for reactions with compounds which are not optically active. Enantiomers may react differently with optically active compounds.
Diastereomers
cis-trans whilst exhibiting optical isomerism
not mirror images
can have multiple chiral centres
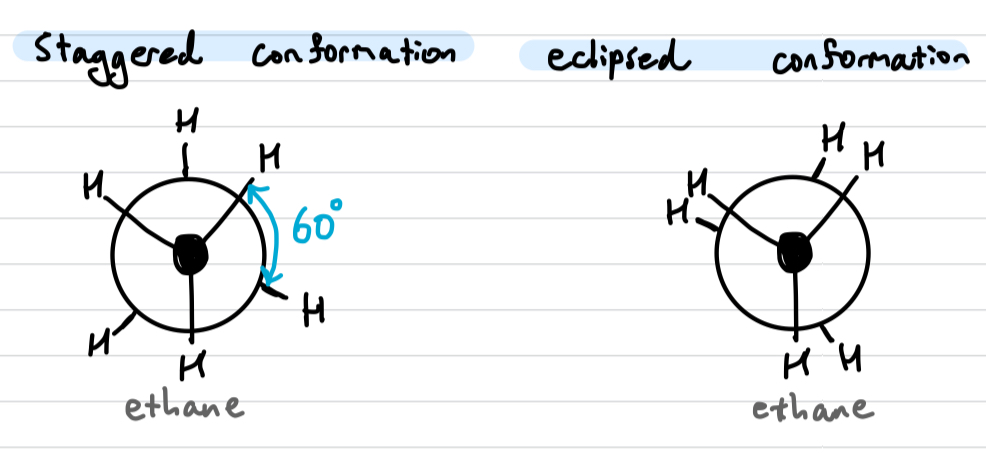
Conformational isomers
rapidly interconverts between staggered and eclipsed conformation at room temperature due to low energy difference
converts via rotation about the single bond
Types of nucleophilic substitution and conditions
Primary halogenoalkanes → Sn2 reaction
Tertiary halogenoalkanes → Sn1
Secondary halogenoalkanes → Sn1 + Sn2 mix
Draw out an Sn2 reaction
if its chiral it inverts like an umbrella
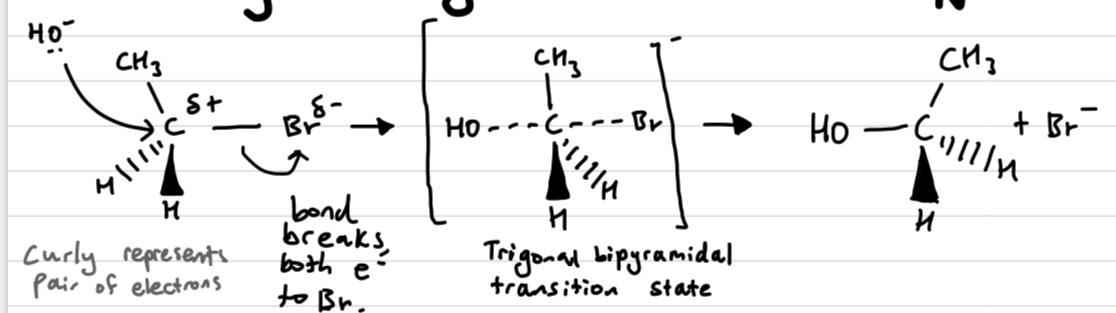
What does bimolecular and unimolecular reaction mean for the rate
Bimolecular - two species are involved in the rate determining step.
Unimolecular - one species involved
Sn2 reactions are bimolecular as the nucleophile and the halogen move in the same step. So the concentration of both species matters - Rate = k[halogenoalkane][nucleophile]
Sn1 is not because only the concentration of the initial species matters. Rate = k[halogenoalkane]
Define steric effect
how readily the compounds can be substituted in regards to ‘space’
energy level diagrams of Sn1 and Sn2 reactions
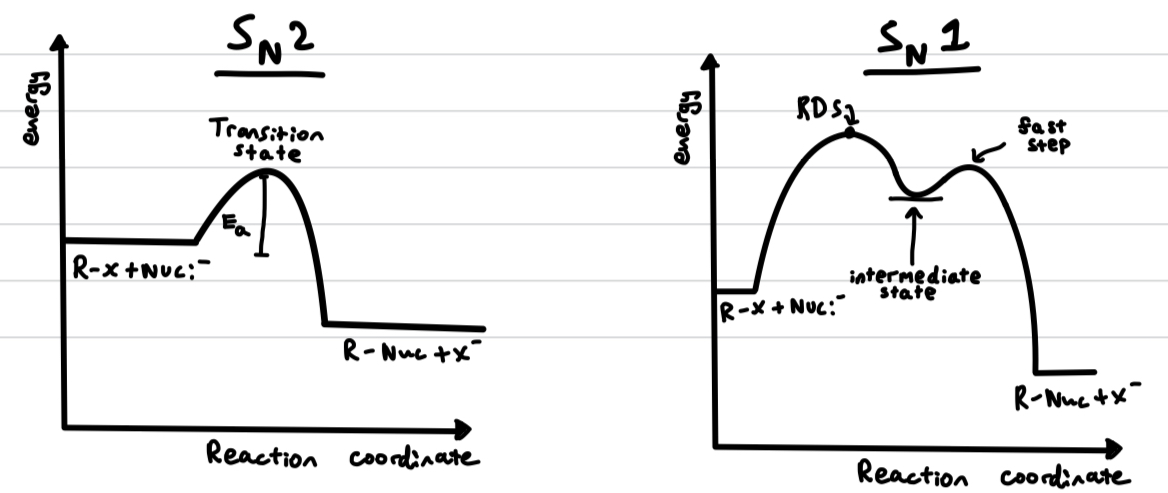
Define heterolytic fission
Covalent bond breaks, electron pair goes to the same side
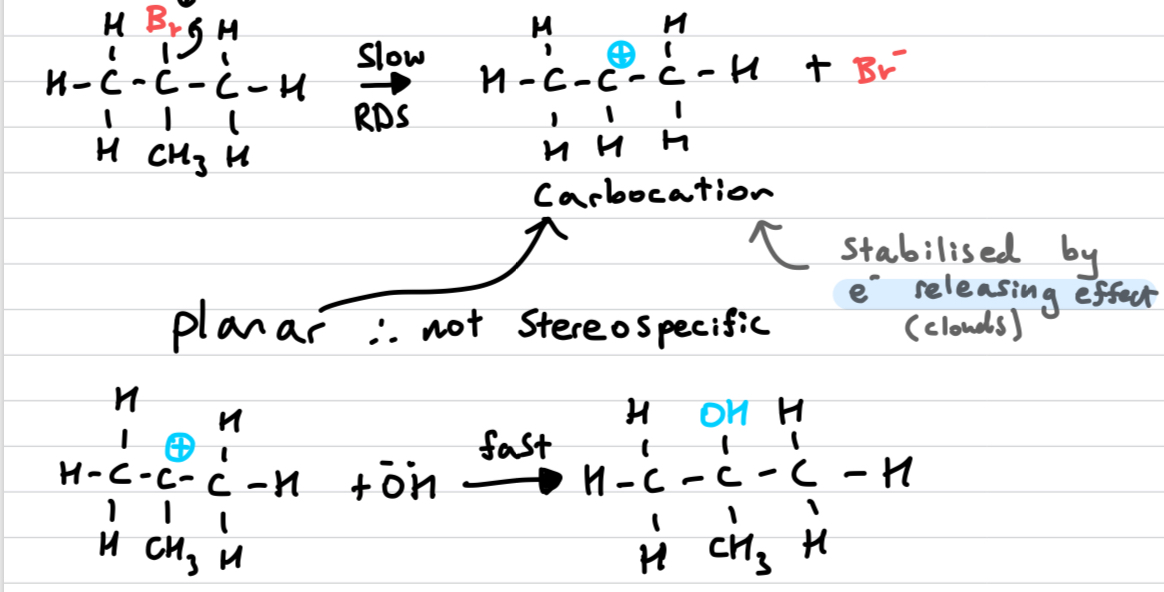
Draw out an Sn1 reaction
REFER TO HETEROLYTIC FISSION WHEN EXPLAINING
What affects the rate of nucleophilic substitution
structure
halogen
nucleophile
solvent
How does struture affect rate of nucleophilic substitution
Sn2: steric effects (space)
Sn1: positive inductive effects stabilises carbocation
How does halogen affect rate of nucleophilic substitution
R-I > R-Br > R-Cl > R-F
higher atomic mass = faster rate of reaction
how does nucleophile affect rate of nucleophilic substitution
Sn2: more negative = faster reaction. e.g OH- > H2O
Sn1: no effect because it is not in the Rate Determining Step (RDS)
What is a polar protic and polar aprotic solvent
Protic - can participate in H-bonding (e.g. water)
Aprotic - can’t participate in H-bonging (e.g. Propanone)
How does solvent affect rate of nucleophilic substitution
Sn1 favoured by protic polar - as it is a good ionizing solvent and thus stabilises the carbocation
Sn2 favoured by aprotic polar - as it is not good at solvating the nucleophile and thus it’s easier to attack the nucleus
Why do alkenes undergo electrophilic addition
120 degree bond angle
double bond is electron dense, therefore attractive to electrophiles

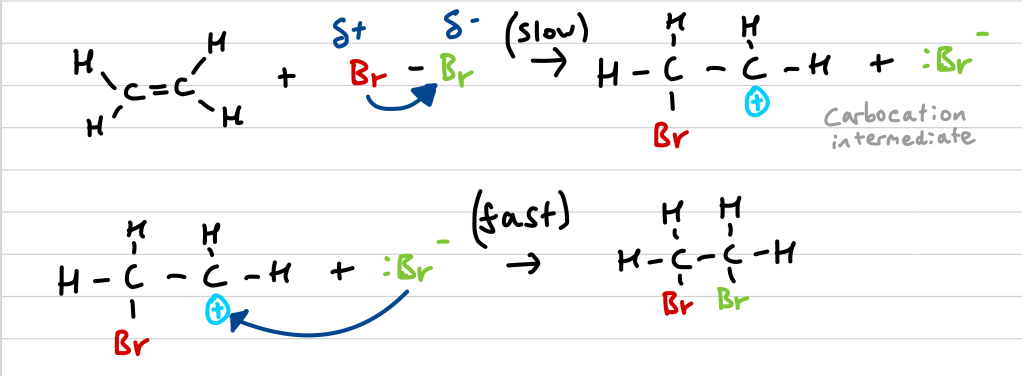
Draw the ethene + bromine mechanism and explain it
bromine is polarised by electron rich double bond
Br2 splits forming Br+ and Br-
Br+ (electrophile) attacks double bond, attaching to it (slow/RDS)
unstable carbocation reacts with Br- (fast)
What is the bromine test used for
determining whether a hydrocarbon is saturated (single bonds) or unsaturated
Draw out the ethene + hydrogen bromide reaction mechanism
similar to ethene + bromine mechanism
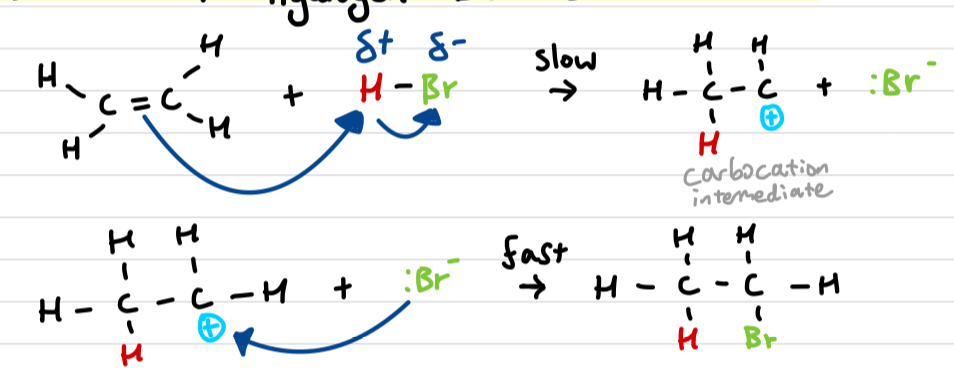
Draw out assymetric electrophilic addition and explain why it occurs
When the double bond is not in the middle, 2 different carbocation intermediates can be formed.
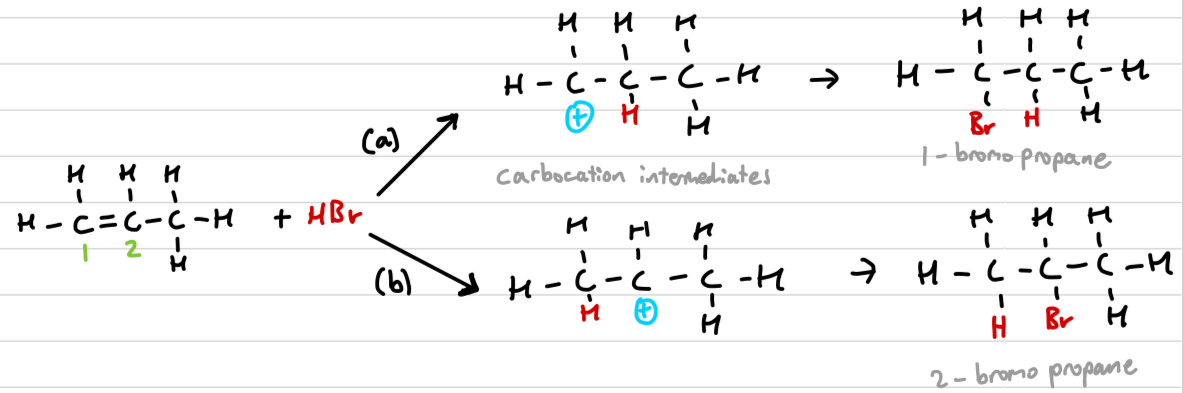
Define positive inductive effects
alkyl group (alkane minus a hydrogen) can push electron density away from themselves
greater positive inductive effects mean the carbocation is more stable, hence mechanism (b) is preffered over (a)
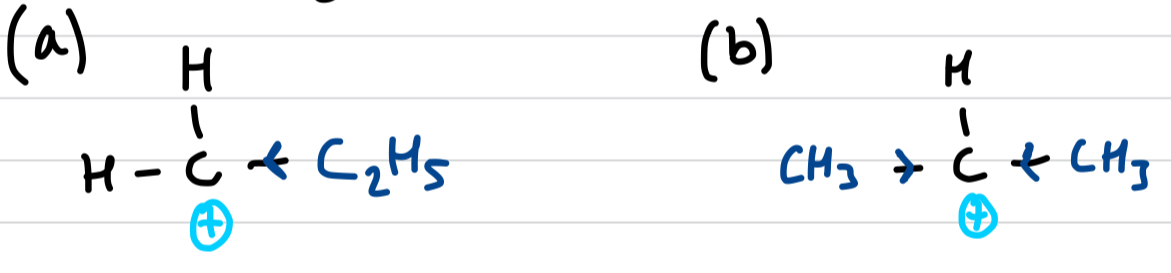
Markovnikov’s rule
The hydrogen will attach to the carbon that is already bonded to the greater number of hydrogens
why does benzene undergo electrophilic substitution
simplest aromatic hydrocarbon compound (or arene)
Carbon to carbon bonds have a bond order of 1.5
delocalised structure of pi bonds around its ring
highly unsaturated, however doesnt behave like other alkanes
highly stable, more likely to undergo substitution (so as to not lose stability from delocalised pi electrons)
ring is electron dense, so it attracts electrophiles
delocalised electrons seek electrophiles, forming a new bond, losing a H → electrophilic substitution
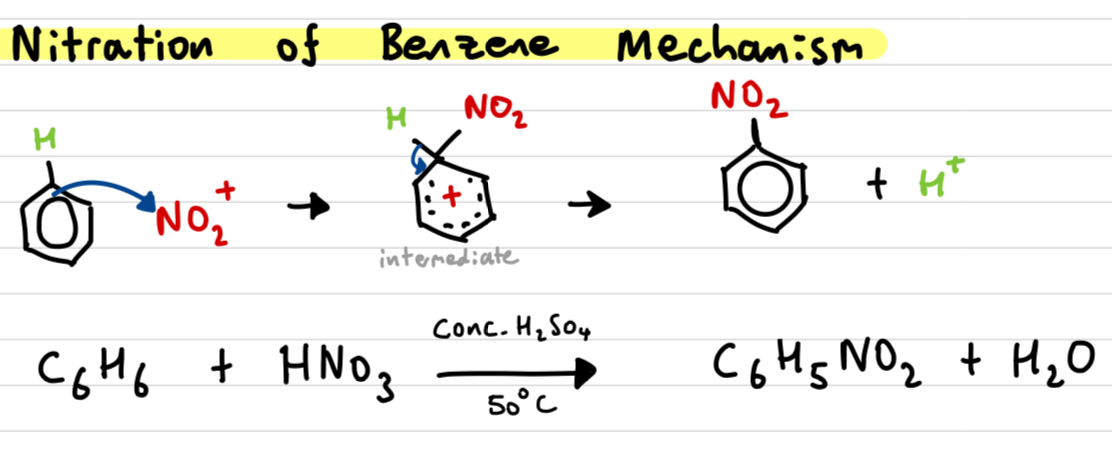
Draw out and explain the Nitration of benzene mechanism
catalyst: Conc. H2SO4 + heat
electron pair of benzene attracted to Nitronium as it is a strong electrophile
Disrupts the delocalised electron ring
NO2+ and hydrogen temporarily attached to unstable carbocation intermediate
electrons from C-H bond are used to reform the arene ring, losing the H+ and forming nitrobenzene (appears as yellow oil)
H+ released reacts with HSO4- to form H2SO4 again
Reduction vs oxidation in organic chem
Most reduced: more hydrogens
Most oxidised: more oxygens
Draw out Reduction reactions of carbonyl compounds
Primary and secondary alcohol oxidation can be reversed by adding reducing agents
all reactions done in acidic conditions
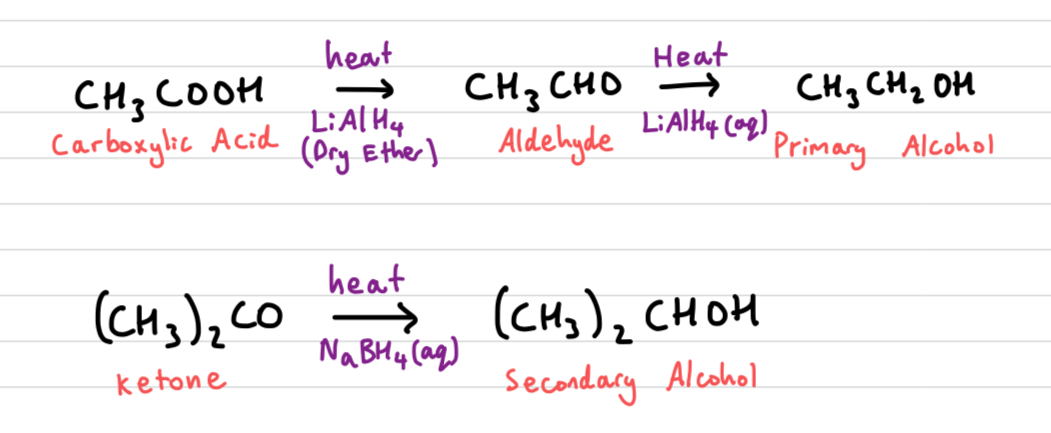
What are the reducing agents for carbonyl compounds
NaBH4 (Sodium borohydride) in aqeous or alcoholic solution, or
LiAlH4 (Lithium aluminium hydride) in anhydrous conditions, e.g. dry ether followed by aqeous acid.
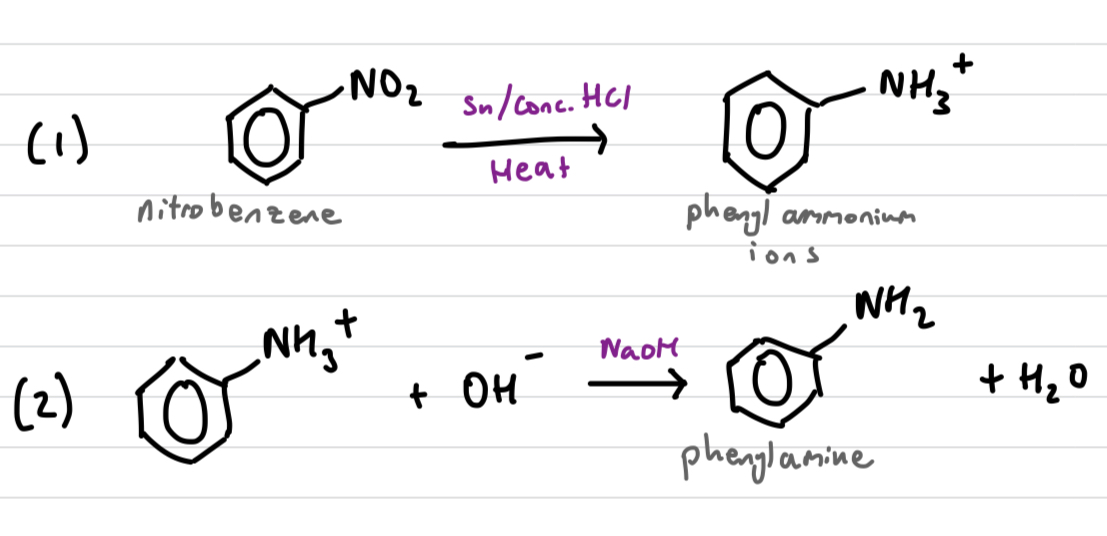
Draw and explain the reduction of nitrobenzene reaction mech-anism
C6H5NO2 (nitrobenzene) can be reduced to C6H5NH2 (phenylamine) in a 2 step process.
C6H5NO2 reacts with a mixture of Sn/Conc. HCL under heat. Acidic conditions protonate the product, phenylammonium ions (C6H5NH3+)
C6H5NH3+ is reacted with NaOH to remove the H+ and form C6H5NH2
Define synthetic routes
series of discrete steps involved in the production of organic compounds
Define retro-synthesis
Working backwards from a desired target molecule
target molecule → precursor → starting materials
What is an electrophile
An electrophile is an electron-deficient species that can accept electron pairs from a nucleophile. Electrophiles are lewis acids.
Explain why a hydroxide is a better nucleophile than water
A hydroxide ion is a better nucleophile than water because it has a negative charge, making it more electron-rich and reactive in nucleophilic reactions. Water is less nucleophilic due to its neutral charge and lower reactivity.
NaOH (aq) + R-X (nucleophilic substitution)
Rate of Sn1 > Sn2
Curly arrows and fishhooks
Heterolytic fission: Curly arrows
Homolytic fission: fish hook
emphasise this on all mechanism diagrams
list halogenoalkanes, alkanes, and alkenes in order of reactivity
Alkenes > Halogenoalkanes > Alkanes
explain distillation and reflux and why its used for alcohols
Distillation: Separates components based on boiling points. Aldehyde (lower boiling point) vaporizes first.
Reflux: Prevents loss of volatile components by condensing vapors back into the reaction mixture.
NaBH4 (sodium borhydride)
catalyst for reducing aldehydes and ketones to primary/secondary alcohols
LiAlH4 (lithium aluminium hydride)
catalyst for reducing carboxylic acids
stronger than NaBH4, cannot be stopped at aldehyde stage, goes straight primary alcohol hole
Anabolism
reaction of synthesis (monomer → polymer) Requires energy
Metabolic pathways
a series of chemical reactions in a cell
Catabolism
Reaction of breakdown (polymer → monomer) Releases energy
Where do most metabolic reations takes place?
highly controlled aqueous environments (since 90% of a cell’s cytoplasm is water)
Factors affecting function and ability to react successfully in metabolic processes
Shape
Structure
Chirality (HL)
Factors that must be maintained to ensure optimal cell function in metabolic process
PH
Temperature
Concentration of components within the cell’s cytoplasm
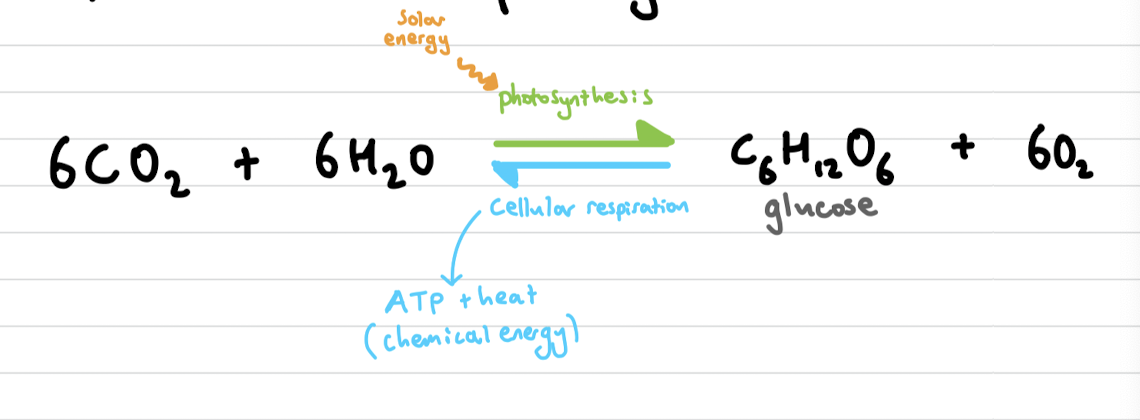
Photosynthesis
The synthesis of energy-rich molecules from carbon dioxide and water using light energy. (endothermic)
Respiration
A complex set of metabolic process providing energy for cells. (exothermic)
Aerobic vs Anaerobic
Aerobic: with oxygen
Anaerobic: in the absence of oxygen
write the equilibrium equation for photosynthesis and cellular respiration
Condensation reaction
produces water from H+ and OH-
Heat and acidic environment is often required
Anabolic
Hydrolysis
water breaking → H2O splits to H+ and OH-
Heat + Acid catalyst/enzymes required
Enzymes ensure it doesn’t reverse
Catabolic
Proteins
biopolymers of 2-amino acids, joined by amide links / peptide bonds
Polypeptide chains
more than 3 amino acids joined by peptide bonds
formation occurs in condensation reactions, reversed in hydrolysis
Isoelectric point
the pH at which a molecule carries no net electrical charge. It is the pH at which a molecule is electrically neutral.
What determines Isoelectric point
R groups. Acidic R groups favour acidic isoelectric point, vice versa.
Zwitterion
A molecule with both positive and negative charges, making it electrically neutral. It forms when an amino acid is at its isoelectric point.
Primary protein structure
sequence of a chain of amino acids
bond: contains peptide bonds
example: polypeptide chains
Secondary protein structure
amino acid folds into a repeating pattern due to H-bonds
bond: Hydrogen bonds between COOH and NH2
example: alpha helix and beta pleated sheets
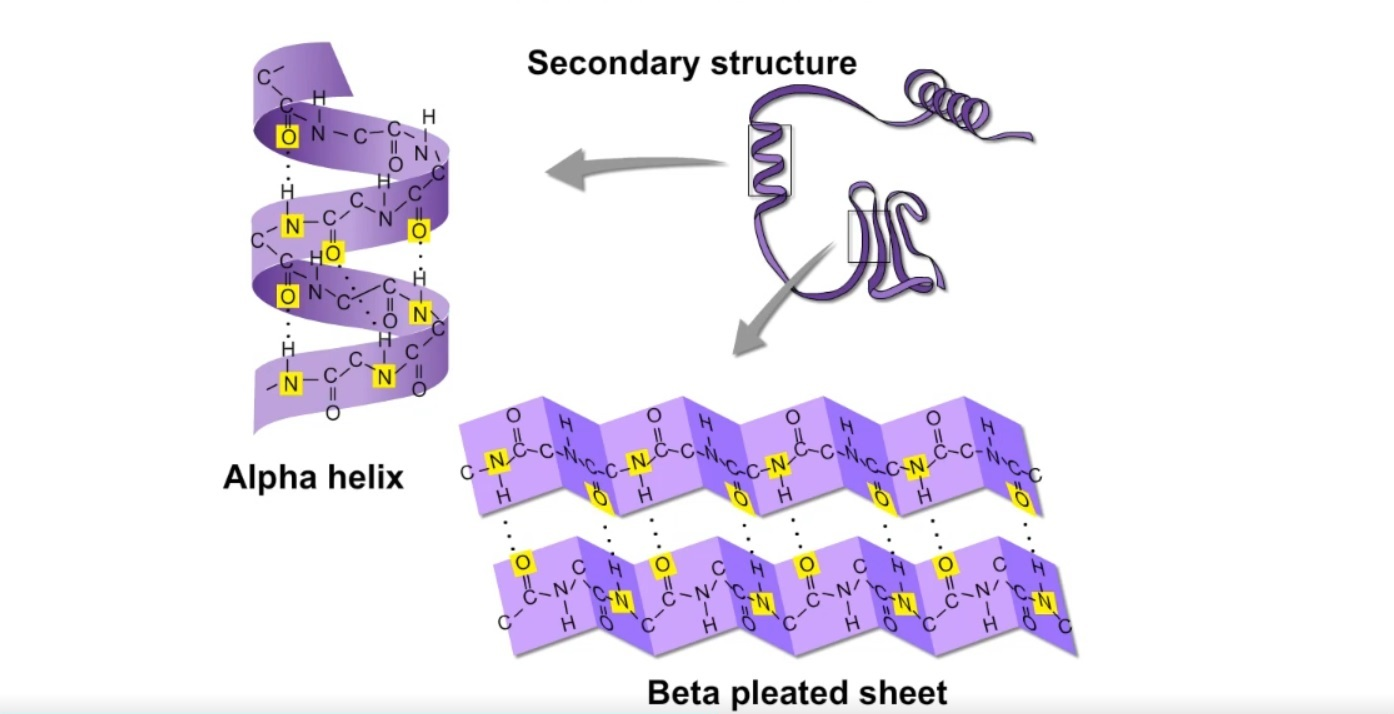
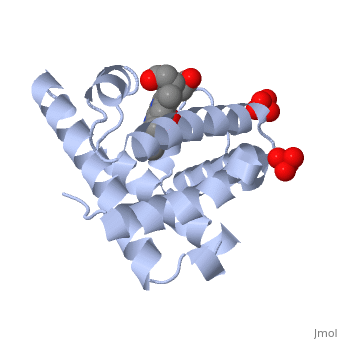
Tertiary protein structure
Three dimensional folding pattern of a protein due to side chain interactions
Bonds: between R-Groups
Hydrogen bonds: helps stabilise protein molecule
Disulfide bonds: strong covalent formed by oxidation of -SH groups in cystein side-chains
LDF: when two molecules are close to eachother they can appear
Ionic bonds: Weak electrostatic interactions
Example: myoblin or enzymes
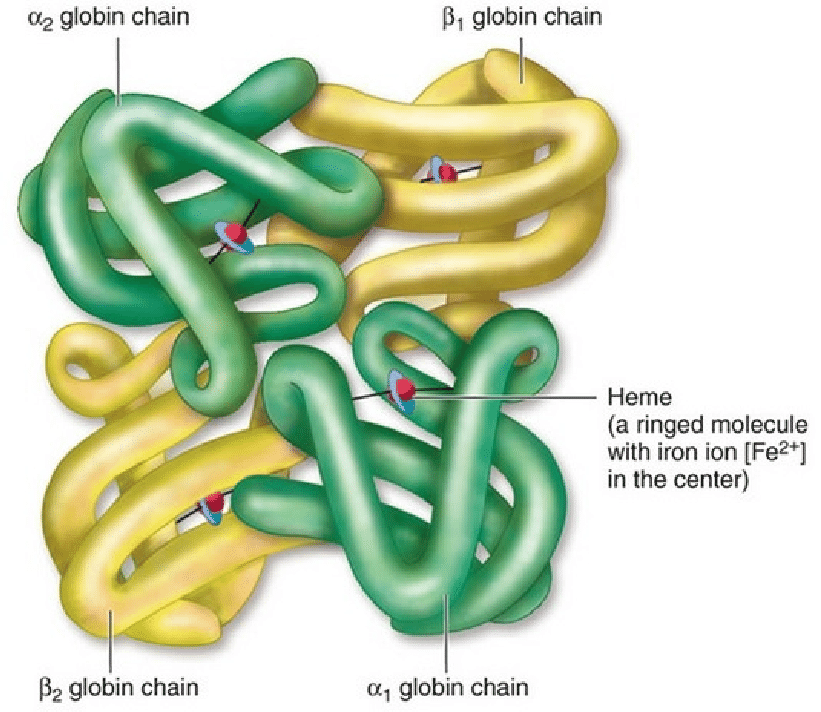
Quarternary protein structures
Proteins consisting of multiple polypeptide chains
Bond: between R-Groups
Example: Haemoglobin
What determines the role of a protein
3D shape determines its role in structural components (e.g. inside cells, tissues, organs, etc) or in metabolic processes
Globular proteins
spherical in shape
soluble in water due to polar R groups being on the outside, whilst non-polar R groups are on the inside
act as chemical messengers (hormones), catalysts (enzymes), and tranpsport molecules.
high temperatures denature the protein by weaking it’s IMF
Fibrous proteins / scleroproteins
Long linear bundles of polypeptide chains, held together by covalent bonds, SS bridge, or H-Bond.
insoluble in water due to exposed R groups being a mix of polar / non polar
Form the basis of structural elements (cells, tissues, etc.) in organisms
not as sensitive to high temperatures, as covalent bonds > IMF
Enzymes
“biological catalysts”
IB: most enzymes are proteins that act as catalysts by binding specifically to a substrate at the active site
usually aqueous as they are homogenous catalysts (same state as other reactants)
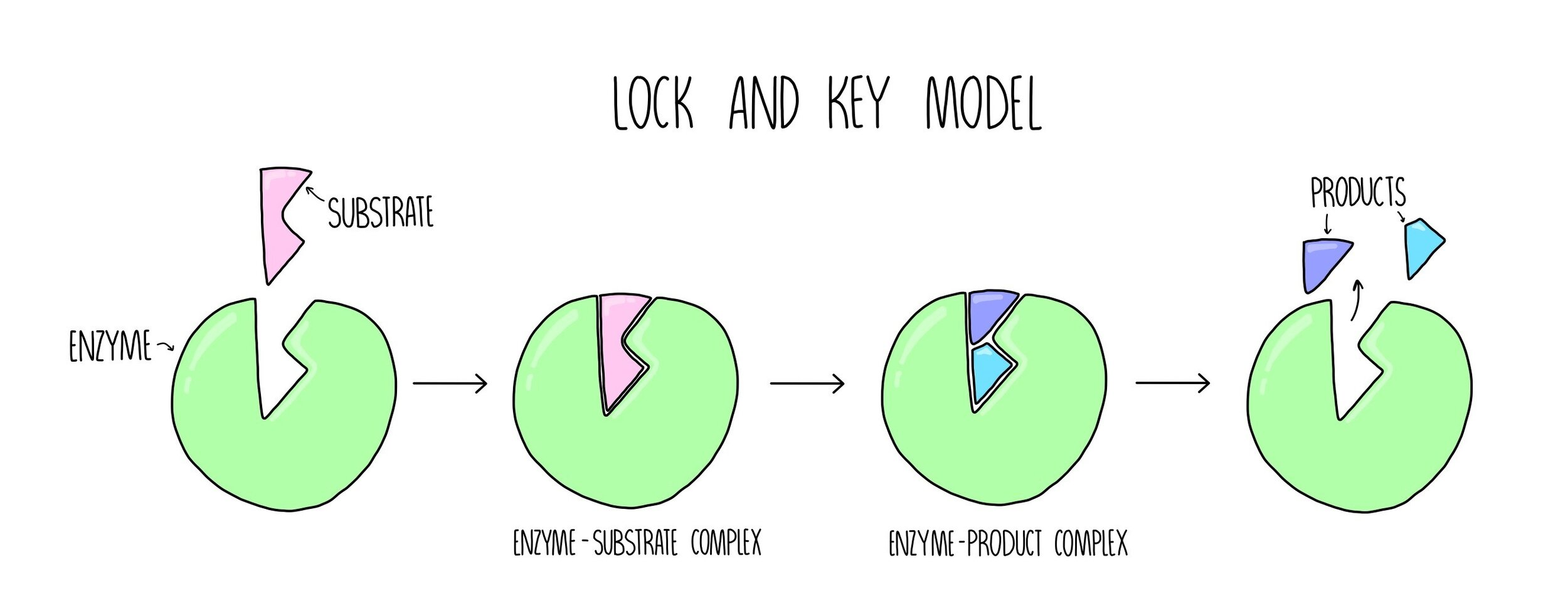
Lock and key model
An enzyme has a cleft in its surface, called the active site. The substrate molecule has a complimentary shape
An enzyme-substrate complex is temporarily formed. The R groups of the amino acids in the active site interact with the substrate.
The substrate is broken apart and the two product molecules leave the active site without damaging the enzyme molecule.
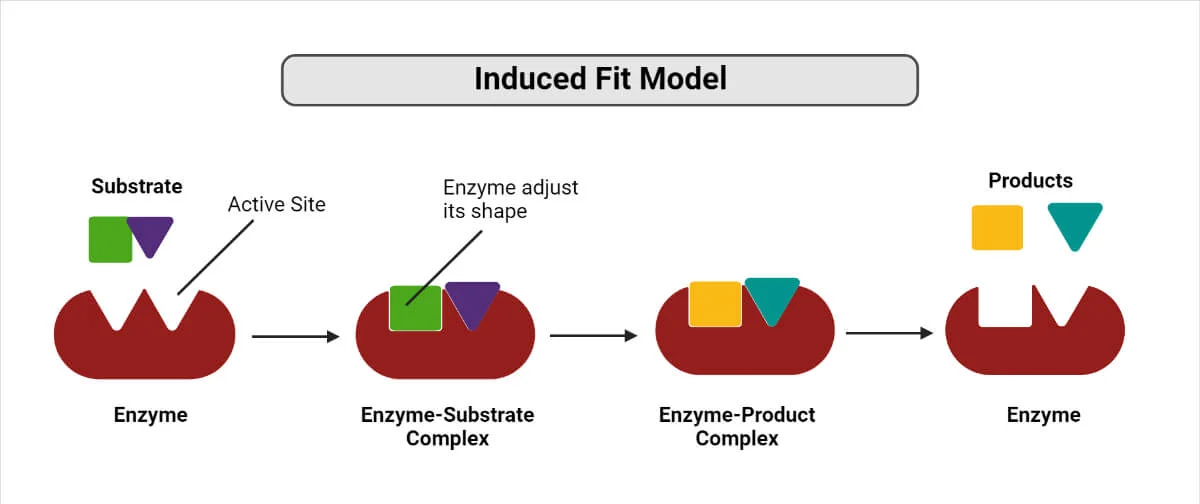
Induced fit model
An enzyme has a cleft in its surface, called the active site. The substrate molecule does not have a complimentary shape
The enzyme changes shape slightly as substrate binds
enzyme-substrate complex is temporarily formed. The R groups of the amino acids in the active site interact with the substrate.
The substrate is broken apart and the two product molecules leave the active site without damaging the enzyme molecule.
What factors affect enzymatic activity
Concentration
Temperature
pH
Heavy metal ions