AQA A-Level - Physical Chemistry
1/320
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
321 Terms
proton
negatively charged particles
relative mass 1
relative charge +1
neutron
relative mass : 1
relative charge: 0
electron, e-
relative mass: 1/2000
relative charge: -1
nuclear symbols
mass number - total number of protons + neutrons
atomic number - total number of protons in nucleus. identifies element.
all atoms of the same element have the same number of protons.
ions have different numbers of protons and electrons
atoms form ions by gaining or losing electrons
negative ions have more electrons than protons
eg: Br- (has 1 more electron than there are protons)
positive ions have fewer electros than protons
Mg2+ --> 2+ charge means there are 2 fewer electrons than protons. Mg has 12 protons so Mg2+ ,must have 10 electrons.
what is an isotope
how are their properties affected?
atoms of same element with different numbers of neutrons
number and arragement of electrons that decides the chemical properties of an element
also do have slightly different physical properties (such as different densities, rate of diffusion, etc)
physical properties tend to depend on mass of the atom
atomic number
number of protons in nucleus - identifies element
all atoms of same element have same number of protons
What did John Dalton propose ? (Model of Atom - 1)
described atoms as solid spheres and different spheres made up different elements
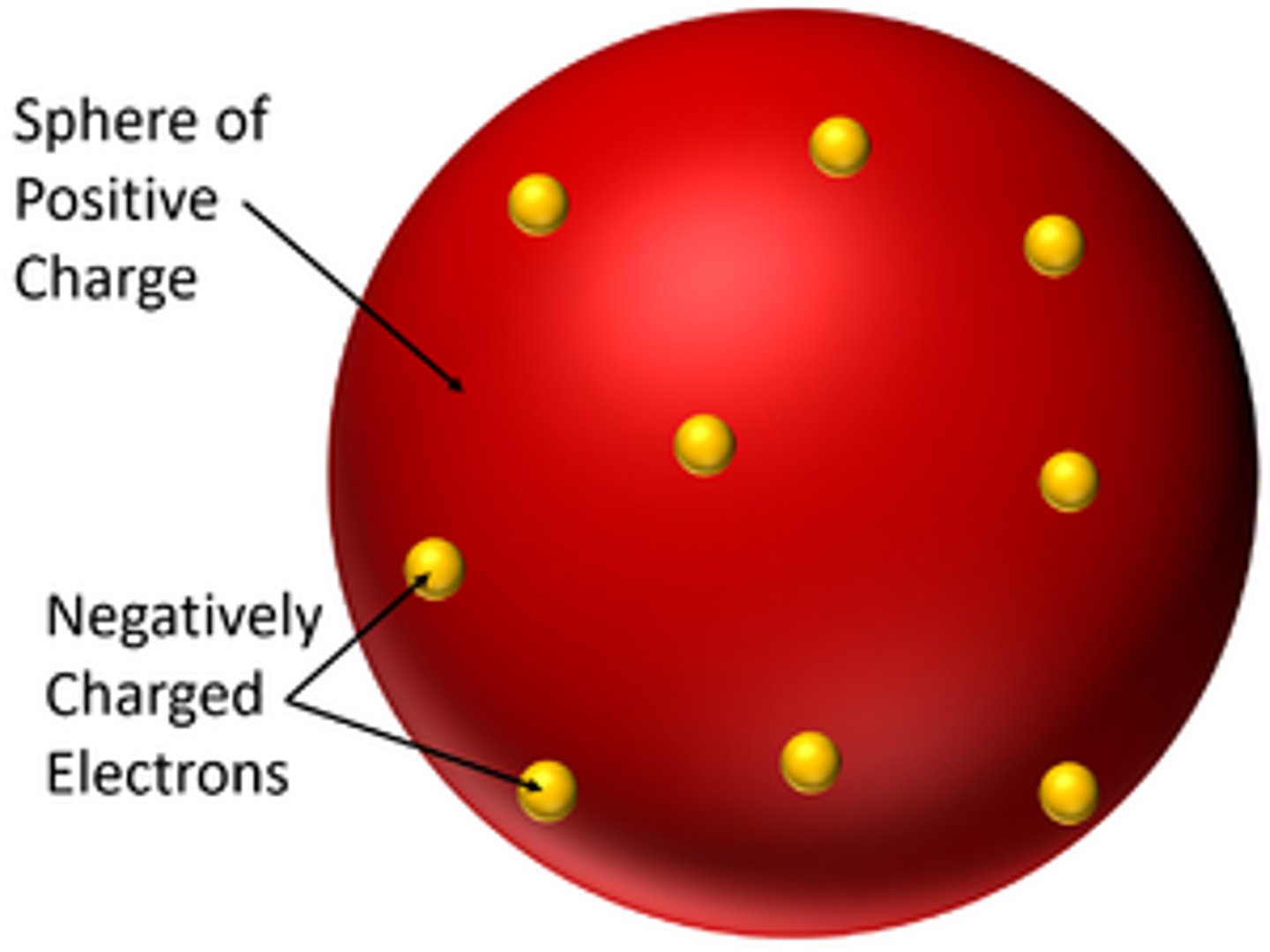
what did J. J. Thomson propose? (Model of Atom - 2)
showed that atoms weren't solid and indivisible
new model was known as plum pudding model
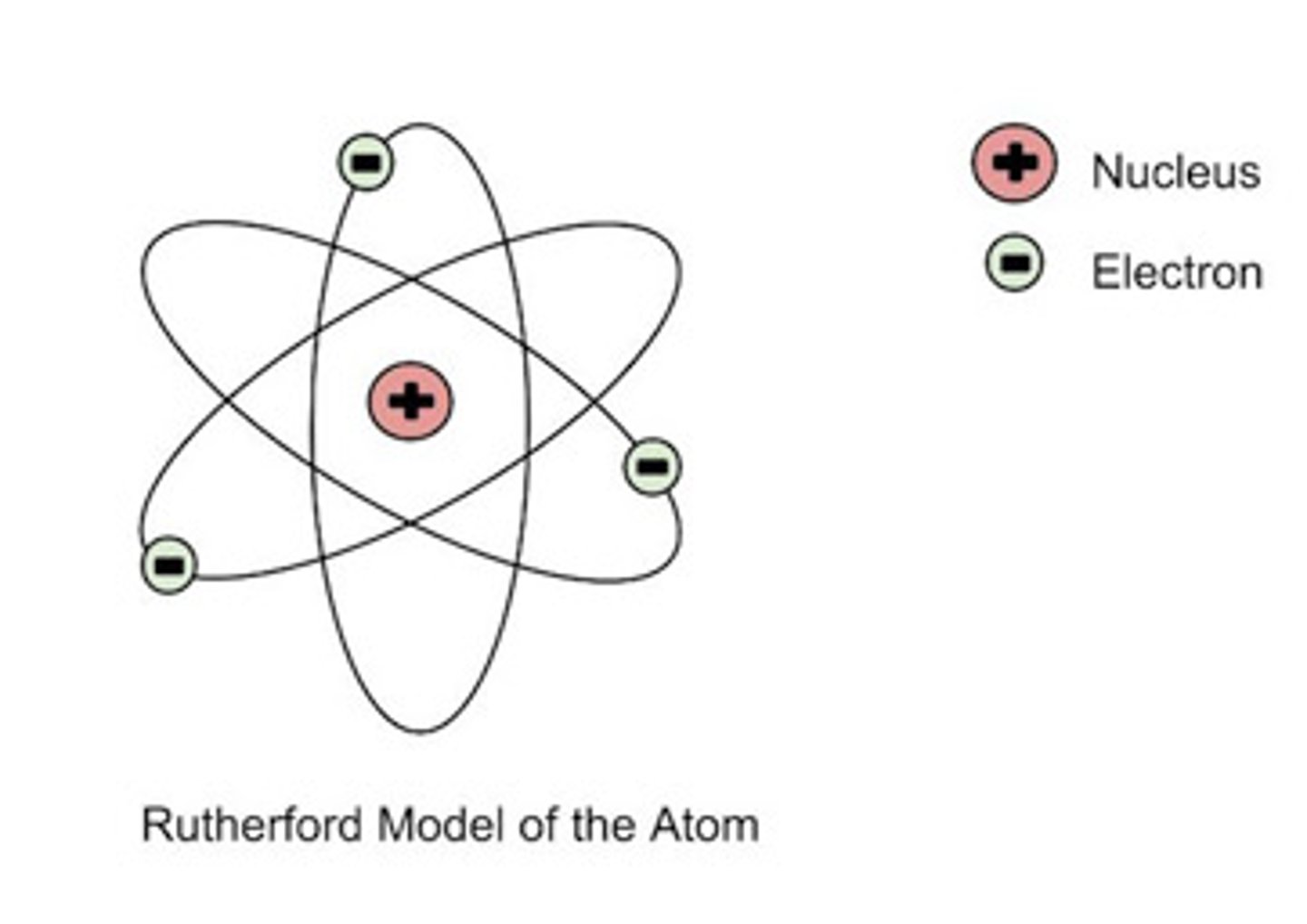
what did Ernest Rutherford propose? (Model of Atom - 3)
rutherford developed nuclear model of atom
tiny + charged nucleus surrounded by cloud of negative electrons
most of atom is empty space
how did Rutherford conduct his Gold Foil Experiment
fired + charged alpha particles at thin sheet of gold
plum pudding suggested alpha particles would be slightly deflected by positive pudding
but most particles passed straight through gold with only a small number being deflected backwards
what did Niels Bohr propose? (Model of Atom - 4)
Bohr suggested electrons exist in shells or obits of fixed energy
when electrons move between shells, electromagnetic radiation is emitted or absorbed
model fitted experimental observations of radiation emitted and absorbed by atoms
what was the Refined Bohr Model set to include? ( Model of Atom - 5 )
scientists later discovered that not all electrons in shell have same energy
refined model to include subshells
Relative Atomic Mass
The average mass of an atom of an element, relative to one-twelfth of the mass of an atom of carbon-12
Relative isotopic mass
The mass of an atom of an isotope compared with one-twelfth of the mass of an atom of carbon-12.
Relative Molecular Mass
average mass of a molecule compared to 1/12th of the mass of a carbon-12 atom
what can a mass spectrometer be used to identify?
what can it be used to determine?
identify elements
determine relative molecular masses
Mass Spec (1) Describe Electrospray Ionization
Electrospray ionization - sample dissolved and pushed through small nozzle at high pressure
high voltage applied to it causing each particle to gain an H+ ion
sample turned into gas made up of positive ions
Mass Spec (1) Describe Electron Impact Ionization
Electron Impact ionization - sample is vaporized and 'electron gun' used to fire high energy electrons at it
> knocks one electron off each particle so they become +1 ions
Mass Spec (2) Describe Acceleration
+ charged ions are accelerated by electric field so they have same kinetic energy
(lighter ions will end up moving faster than heavier ions)
Mass Spec (3) Describe Ion Drift
ions enter a region with no electric field so they drift through it
lighter ions will drift through faster than heavier ones
Mass Spec (4) Describe Detection
detectors detect charged particles and mass spectrum produced
an electrical current is produced in detector when a charged particle hits it
lighter ions travel at higher speeds in drift region they reach the detector in less time than heavier ions
Exam Technique: Mass Spectrum
A graph with % abundance plotted against mass/charge, gained as a result from the mass spectrometer.
if the sample is an element, each line will represent a different isotope of the element
height of each peak gives relative isotopic abundance
Mass Spectrum with Electrospray Ionization
a H+ would have been added to each particle to form +1 ions -
so mass/charge ratio of each peak would be one unit greater than relative mass of each isotope
Mass Spectrum with Electron Impact Ionization
one electron has been knocked off each particle to turn them into +1 ions
so m/z ratio of each peak is same as relative mass of that isotope
How to calculate Ar (Relative Atomic Mass) from a Mass Spectrum
for each peak read the % abundance for the y axis and relative isotopic mass from x axis
multiply them together to get total mass of each isotope
add up all totals
divide by 100
Exam technique: If Relative Abundance isn't given as a percentage
multiply abundance and m/z together to get total mass of each isotope
add them together
then divide them by the sum of relative abundances (addition)
instead of 100
How is Mass Spectrometry used to Identify Elements
elements with different isotopes produce more than one line in mass spectrum since isotopes have different masses
can be used as 'fingerprints' to identify certain elements
(eg % abundance of one isotope may be 79 so its 24Mg, other is 10% so its 25Mg)
how is Mass Spectrometry used to Identify Molecules
molecular ion, M+ formed in mass spectrometer when one elctron is removed from the molecules
gives a peak int he spectrum with a m/z equal to Mr of molecule
can help identify unknown compound
Describe how electrons move around the nucleus.
electrons have fixed energies
move around nucleus in shells
the further a shell is from nucleus, higher its energy
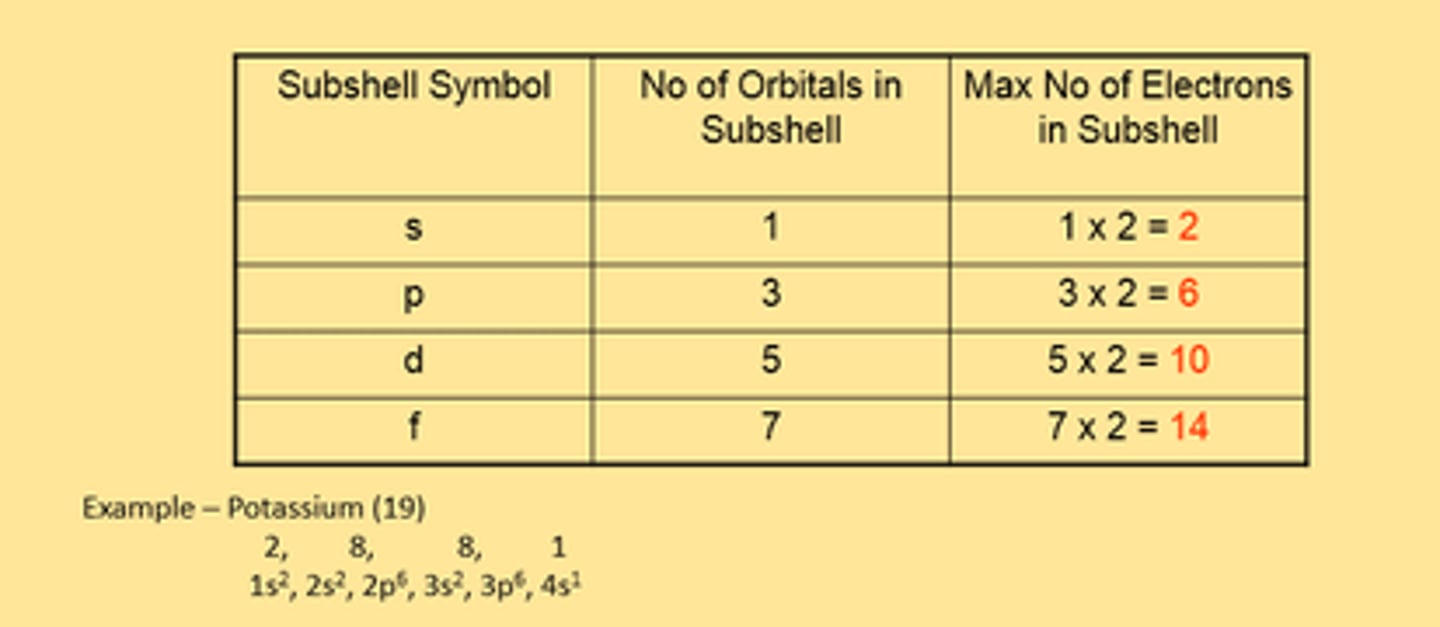
Electron Sub-shell + Orbital Table
two electrons in each orbital spin in opposite directions
Explain the structure of Transition Metals and Electronic Structure
Chromium + Copper donate one of their 4s subshells to 3d subshells
They're 'happier' with a more stable full or half-full d subshell
when they become ions they lose their 4s electrons before their 3d
(since it comes before 3d on periodic table)
Exam Technique: Electronic Structure Decides Chemical Properties of Element (S,P,D,0)
S block elements (group 1 + 2) have 1/2 outer shell electrons
easily lost to form positive ions with inert gas configuration
(eg Na = 1s2s2p3s --> Na+ = 1s2s2p6)
P block elements (group 5,6 +7) can gain 1, 2 or 3 electrons to form negative ions with inert gas configuration
(O: 1s2s2p4 --> O2-: 1s2s2p6)
D block elements (transition metals) tend to lose s and d electrons to form positive ions
Group 0 (inert gases) - completely filled s and p sub-shells so no need to gain or lose/share electrons - they are inert because of full subshells
what is First ionization energy
energy needed to remove 1 electron from each atom in 1 mole of gaseous atoms to form 1 mole of gaseous 1+ ions
Exam Technique: Important points about ionization energies
1) must use gas state symbol (g) since ionization energies are measured for gaseous atoms
2) Always refer to 1 mole of atoms, rather than to a single atom
3) Lower the ionization energy, the easier to form an ion
High Ionization Energy (Factors affecting Ionization Energy)
high ionization energy means theres a high attraction between electron + nucleus so more energy is needed to remove electon
Shielding (Factors affecting Ionization Energy)
lessening of the pull of nucleus by inner shells of electrons.
as number of electrons between outer electrons and nucleus increases
outer electrons feel less attraction towards nuclear charge
Nuclear Charge (Factors affecting Ionization Energy)
more protons in nucleus, the more positively charged the nucleus is and the stronger the attraction is for electrons
Distance from Nucleus (Factors affecting Ionization Energy)
attraction falls off very rapidly with distance
electron close to nucleus will be much more strongly attracted than one further away
Successive Ionization energy
A measure of the energy required to remove each electron from an atom, leaving only the nucleus.
Second Ionization Energy
energy needed to remove 1 electron from each ion in 1 mole of gaseous 1+ ions to form 1 mole of gaseous 2+ ions
*Exam Tech: Writing Equations for nth Ionization Energy
X(^N-1)+ --> X^n+ + e^-
How do Successive Ionization Energies give evidence for sub-shell Structure
within each shell successive ionization energies increase
electrons are being removed from an increasingly positive ion so there's less repulsion amongst remaining electrons
so they are held more strongly by the nucleus
big jumps in ionization energy happen when a new shell is broken into - electron being removed from a shell closer to nucleus
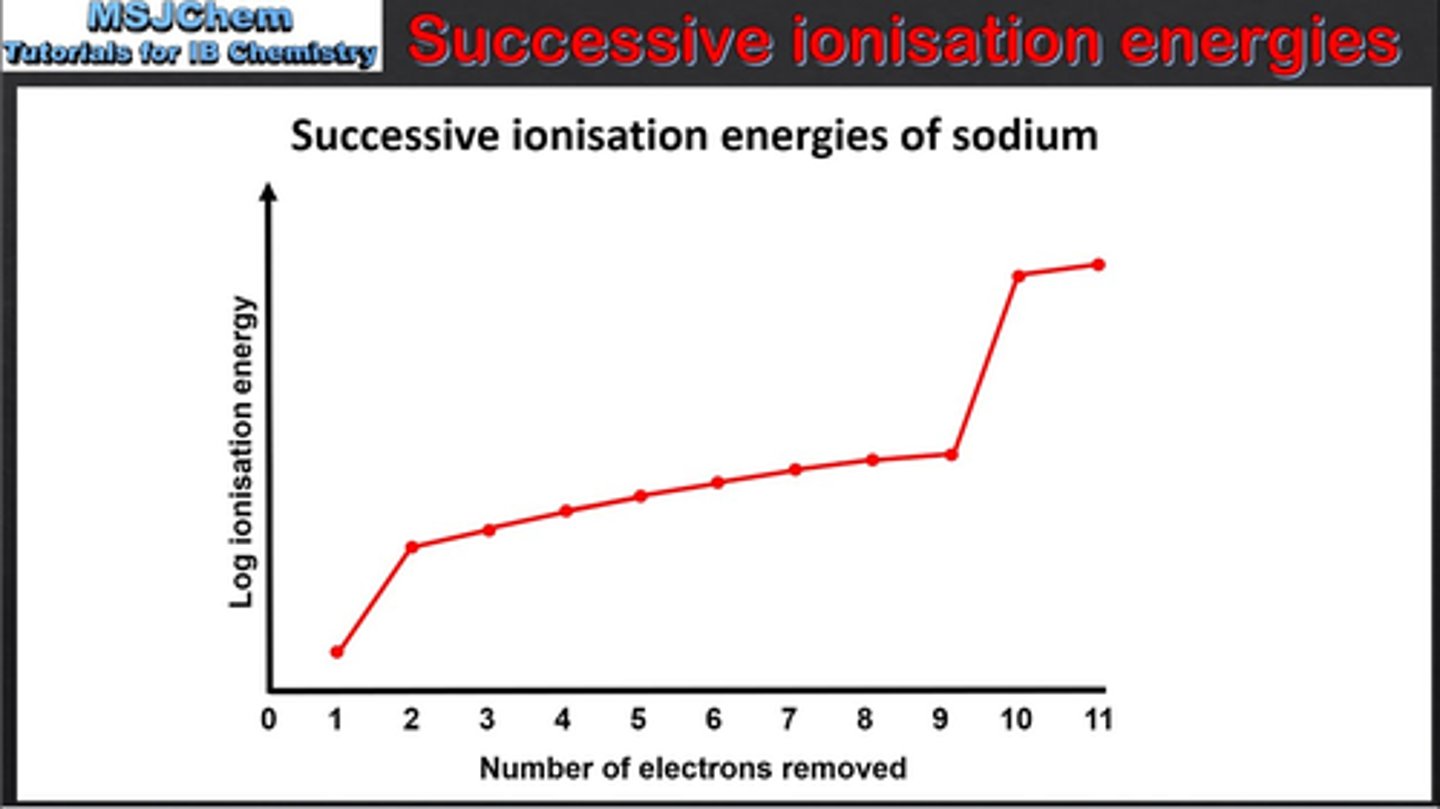
Exam technique: How to identify Periodic Table Group Number from Graph of Successive Ionization Energies
count how many electrons removed before big jump to find group number
(eg in sodium, one electron is removed before first big jump)
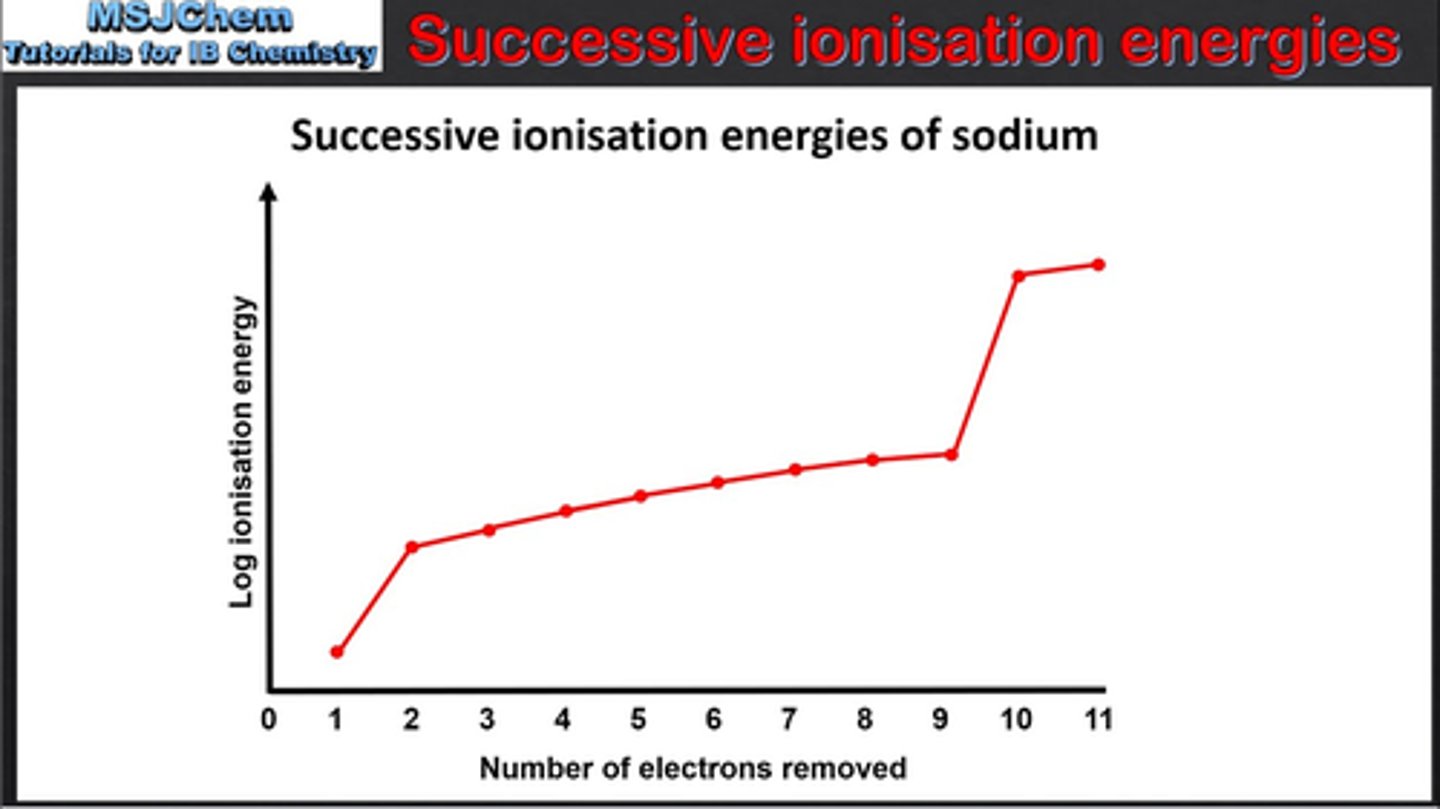
Exam Technique: How to identify Electronic Structure from Graph of Successive Ionization Energies
can be used to predict electronic structure of elements
working from RIGHT TO LEFT
count how many electrons in each shell, starting from the first
What is the Trend in First Ionization Energies
the first ionization energies down a group decrease
the first ionization energies across a period generally increase
Why does Ionization Energy Decrease Down Group 2
each element down group 2 has an extra electron shell compared to the one above
so extra inner shells will shield the outer electrons
extra shells mean that outer electrons are further away from nucleus so the nucleus's attraction will be greatly reduced
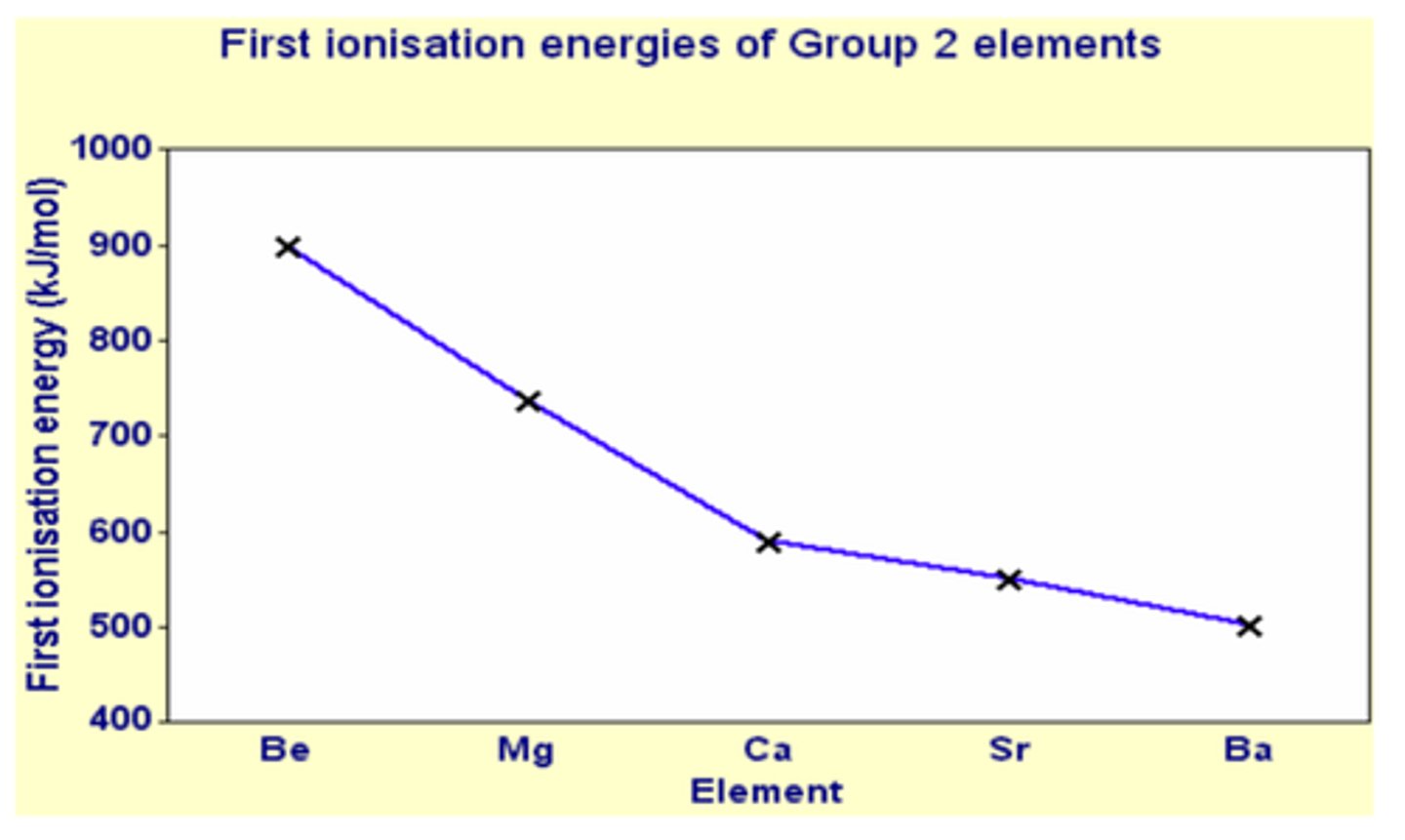
Why does Ionization Energy Increase Across a Period
ionization energies increase across a period
since number of protons is increasing so a stronger nuclear attraction
extra electrons are roughly the same energy level even if outer electrons are in different orbital types
means there are generally little extra shielding effect/distance to lessen attraction from nucleus
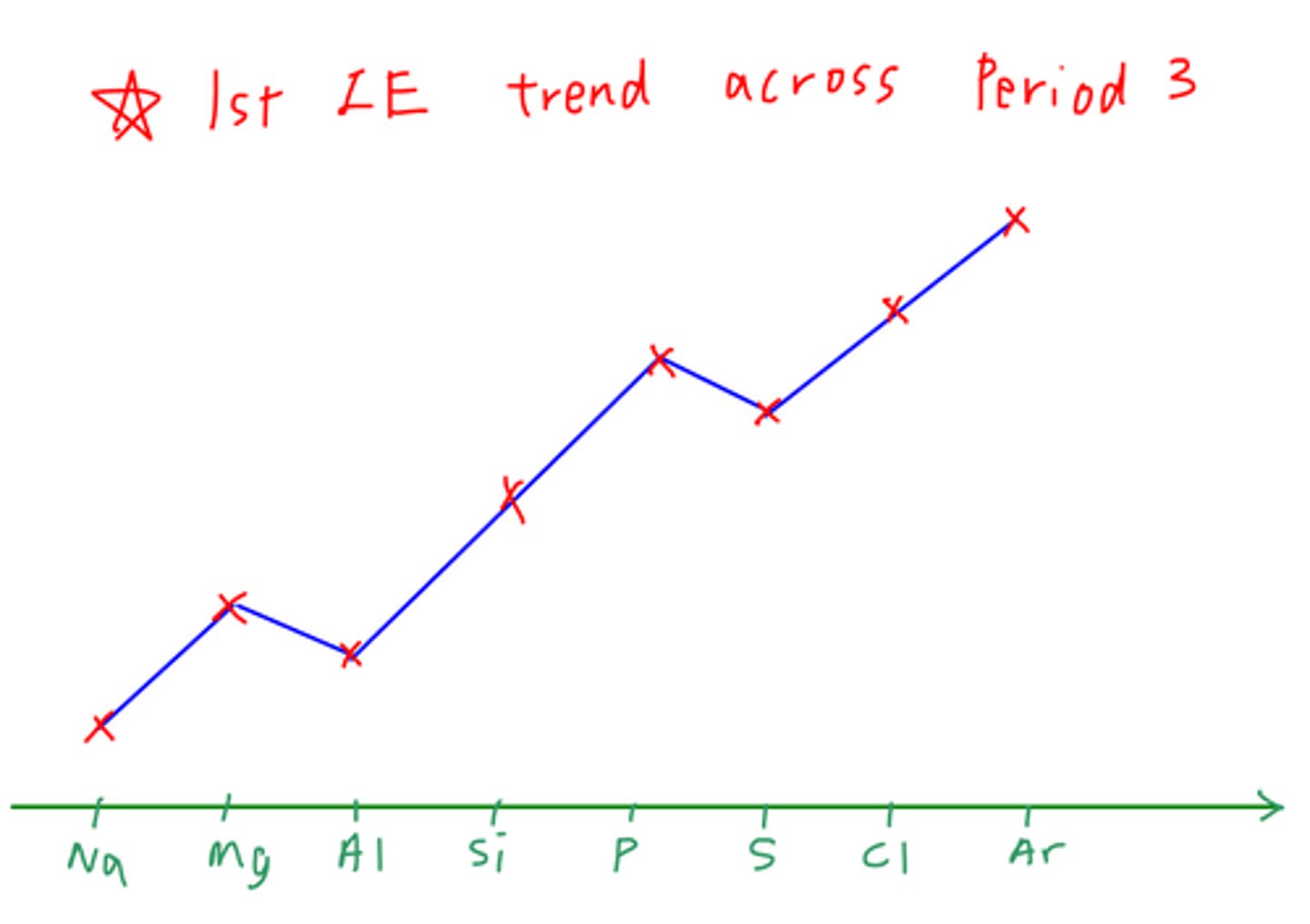
Why do drops between groups 2 and 3 shows sub shell structure (Evidence for Theory of Subshells)
eg: Be and B
Outer electron of group 3 is in a 'p' orbital rather than a 's'.
p orbital has a slightly higher energy than the s orbital so the electron is further from the nucleus
p orbital has additional shielding provided by s2 electrons
both factors together are strong enough to override effect of increased nuclear charge, resulting in ionization energy dropping slightly
Why does the drop between groups 5 and 6 provide evidence for Electronic Structure Model?
eg: P and S
shielding is identical to both atoms and electron is being removed from identical orbital
in phosphorus - electron being removed from a slightly occupied orbital (3p3)
in sulfur electron is being removed from orbital containing 2 electrons (3p4)
repulsion between 2 electrons means electrons are easier to remove from shared orbitals
Number of particles equation
number of particles = number of moles x Avogadro's constant
Amount of a substance in moles Equation
Number of moles = mass of substance / Mr
Concentration of a Solution equation + units
concentration = mol dm-3
number of moles = conc (mol dm-3) x volume (dm3)
*Exam Technique: Different Units provided
You may be provided a solution that's in cm3 instead of dm3 and told to calculate the mass of a compound.
So, you convert the units to dm3
Calculate the number of moles (m=c x v)
and then
mass = number of moles x Mr
Ideal Gas Equation
PV=nRT
where:
p = pressure (Pa)
V= volume (m3)
n = number of moles
R = the molar gas constant
T = temperature. (K = °C + 273)
ensure everything is in the CORRECT units when you conduct calculations
*Exam Technique: Balancing Equations
1) work out how many of each atom you have on each side
you don't need to have full amounts (as in you can do 1 1/2 mol)
*Exam Techniques: Writing Ionic Equations
in an ionic equation, only the reacting particles (and products they form) are included
ionic substances in the equation will dissolve, breaking up into ions in solution
so eg:
the full balanced inital equation
> HNO3 + NaOH --> HaNO3 + H2O
rewritten equation with all of the ions that are in the reaction mixture
> H+ + NO3- + Na+ + OH- --> Na+ NO3- + H2O
cross out any ions that appear on both sides of the equation
H+ + OH- --> H2O
make sure all the charges are balanced and ✔
Empirical Formula and Molecular Formula
empirical formula - gives the simplest whole number ratio of atoms of each element in a compound
molecular formula - gives the actual number of atoms of each element in a compound
> made up of a whole number of empirical units
*Exam Techniques: Empirical Formula Calculations
1) will be provided with empirical formula and ACTUAL mR and having to find the molecular formula.
calculate the empirical mass of molecule and then compare with Mr & multiply by common multiple
--
2) may be told to work out empirical formula of a compound from percentages of different elements it contains
"a compound is found to have % composition of ......"
in 100g, divide its mass by the mr
and then divide it by the smallest figure of numbers in elements
that gives the ratio, which gives you the empircal formula
Theoretical Yield
the maximum amount of product that can be produced from a given amount of reactant
assumes no chemicals are lost in the process.
Actual Yield
actual mass of product (the actual yield) will always be less than the theoretical yield.
many reasons for this, e.g not all the 'starting' chemicals reacting fully
Percentage Yield Equation
percentage yield = actual yield/theoretical yield x 100
What is the purpose of percentage yield
it tells you how wasteful a process is, based on how much of the product is lost during the process
however it doesn't measure how wasteful the reaction is.
100% yield could still be wasteful if the a lot of the atoms of the reactants are by-products
What is Atom Economy
measure of the proportion of reactant atoms that become part of the desired product (rather than by-product) in balanced chemical equation
Atom Economy Equation
Mr of desired product/Mr of all products x 100
Importance of Atom Economies
companies in chemical industry try to use processes with high atom economies
processes with higher atom economies are better for environment since they produce less waste.
> any waste made needs to be disposed of safely, so the less made the better
make more efficient use of raw materials so they are more sustainable (use up natural resources slowly)
less expensive, companies will have to spend less on separating desired product from waste products + treating waste
Ionic Bonding
electrostatic attraction holds positive and negative ions together - it's very strong.
simplest ions are single atoms that have lost/gained electrons so they have a full outer shell
Compound Ions
ions made up of groups of atoms with an overall charge
Examples:
Sulfate: SO4^2-
Hydroxide: OH-
Nitrate: NO3-
Carbonate: CO3^2-
Ammonium: NH4 +
*Exam Technique: How to figure out formula of an ionic compound
overall charge of any compound is 0
so all the negative charged in the compound must balance all the positive charges
Giant Ionic Lattice (Why is it called "giant" , what is a lattice + example)
lattice - regular structure
structure is called 'giant' since its made up of the same basic unit repeated over and over again
Example: Sodium Chloride
> Na+ and Cl- ions are packed together. Lattice is cube shape.
Ionic Compound Properties (Conductivity)
conduct electricity when molten or dissolved - but not when they're solid
> ions are free to move in a liquid and they carry a charge
> in a solid the ions are fixed in position by strong ionic bonds
Ionic Compound Properties (Melting Point)
have high melting points
> giant ionic lattices held together by strong electrostatic force
> takes loads of energy to overcome these force so melting forces are very high
Ionic Compound Properties (Polarity)
tend to dissolve in water
water molecules are polar - part of molecule has small negative charge and other bits have small positive charges
charged parts pull ions away from the lattice causing it to dissolve
Define Molecule + bonding in molecules
molecules form when two or more atoms bond together
held together by strong covalent bonds
(e.g chlorine gas, carbon monoxide + ethanol are all molecules)
Covalent Bonds
bonds creates by two (or more) atoms sharing electrons so they've both got full outer shells of electrons
happens between non metals
both of the positive nuclei are attracted electrostatically to the shared electrons.
Giant Covalent Structure (Macromolecular)
huge network of covalently bonded atoms
carbon atoms can form this type of structure since they can each form four strong covalent bonds
(e.g graphite + diamond)
Structure of Graphite
carbon atoms arranged in sheets of flat hexagons covalently bonded with 3 bonds each
fourth outer electron of each carbon is delocalised
weak bond between layers in graphite are easily broken so the sheets can slide over each other
delocalized electrons in graphite aren't attracted to any particular carbon atoms so they are free to move along the sheets carrying a charge
layers are quite far apart compared to the length of the covalent bonds so graphite has a low density
Properties of Graphite
> an electrical conductor
due to strong covalent bonds in hexagon sheets, graphite has a very high melting point (over 3900K)
insoluble in any solvent - covalent bonds in sheets are too strong to break
has a low density and used to make lightweight strong sports equipment
Diamond Structure
made up of carbon atoms
each carbon covalently bonded to four other carbon atoms.
atoms arranged into a tetrahedral shape
Diamond Properties
due to strong covalent bonds:
> diamond has very high melting point
> is extremely hard - used in diamond-tipped drills + saws
> vibrations travel easily through stiff lattice so its a good thermal conductor
> can't conduct electricity - all outer electrons held in localised bonds
> diamond won't dissolve in any solvnet
> cut diamond to form gemstones. structure makes it refract light which is why it sparkles
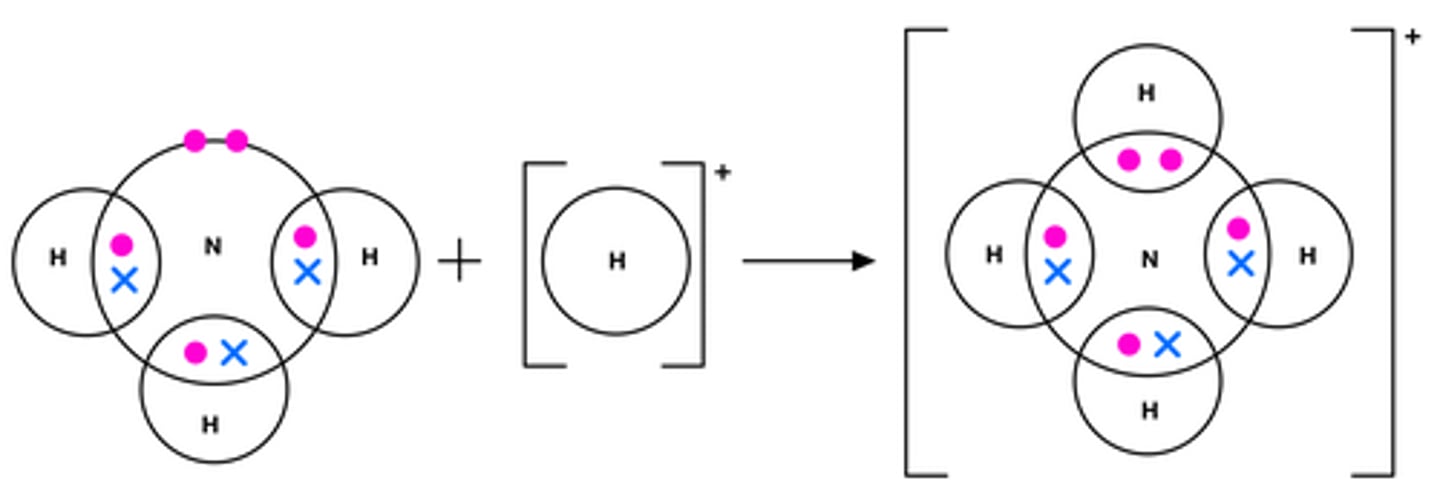
Dative Covalent Bonding
Where both electrons are provided by one atom for a covalent bond.
Charge Clouds
bonding pairs and lone pairs of electrons
area where you have a really big chance of finding an electron pair
electrons don't stay still - whizz around charge cloud
Bonding Pairs and Lone Pairs repel each other
electrons all negatively charged so charge clouds will repel each other as much as they can
+
pairs of electrons in outer shell of an atom will sit as far apart from each other as they can
Valence-Shell Electron Pair Repulsion Theory
shape of charge cloud affects how much it repels other charge clouds
greatest angles are between lone pairs of electrons
+
bond angles between bonding pairs often reduced since they are pushed together by lone pair repulsion
3 types of VSEPR theory angles
Lone-pair / lone-pair angles are the biggest
Lone-pair / bonding pair angles are the second biggest
Bonding pair/bonding pair angles are the smallest
Exam Technique: Using Number of Electron Pairs to predict the shape of a molecule
work out which central atom
use periodic table to work out number of electrons in outer shell of the central atom
add one to this number for every atom the central carbon is bonded to
divide by 2 to find the electron pairs on the central atom
compare number of electron pairs to the number of bonds to find the number of lone pairs and the number of bonding pairs on the central atom
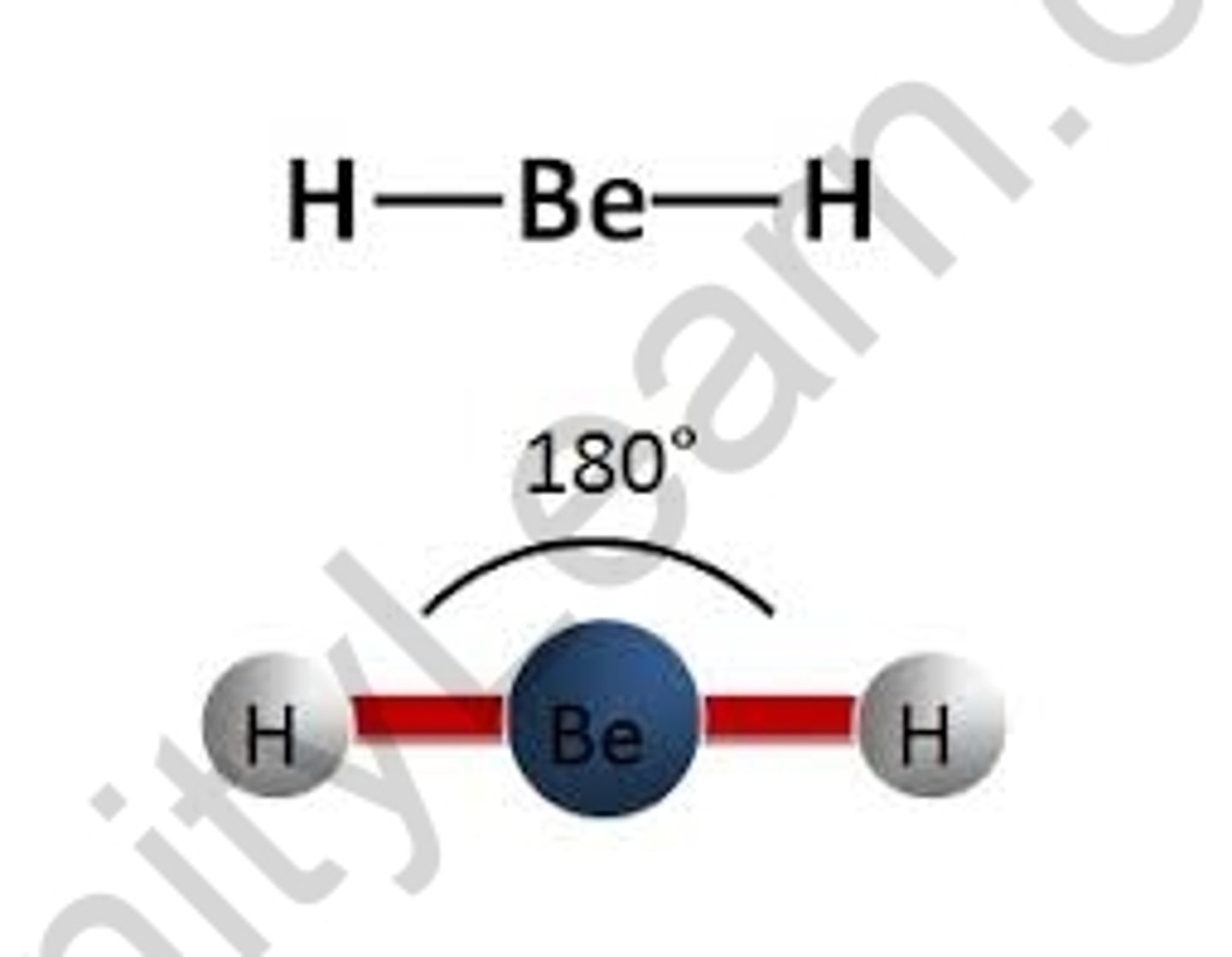
Shape of molecule: 2 electron pairs
no lone pairs
linear
180°
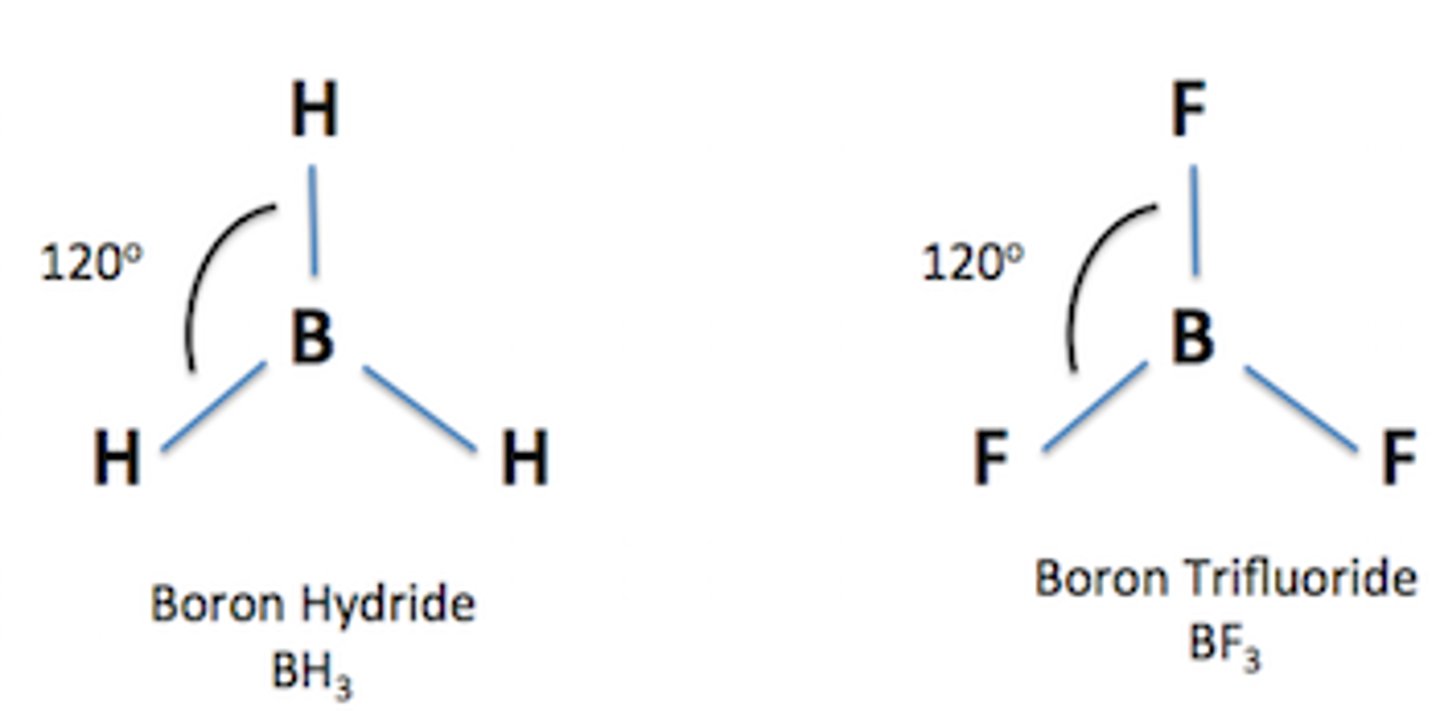
Shape of molecule: 3 electron pairs
no lone pairs
trigonal planar
120°
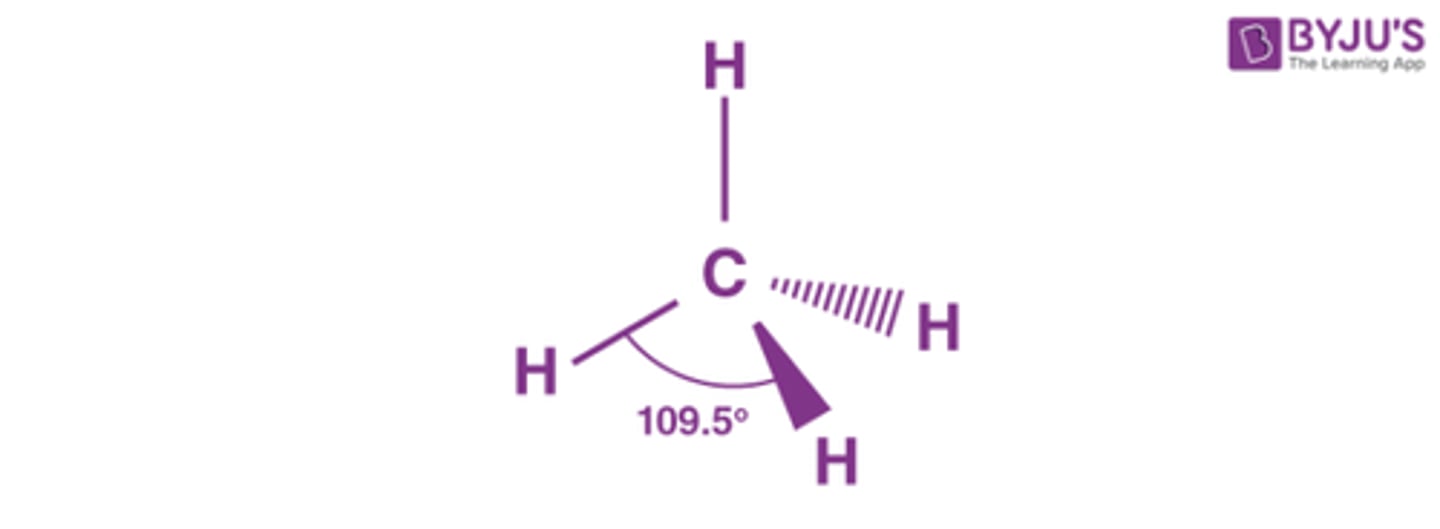
Shape of molecule: 4 electron pairs, no lone pairs
no lone pairs
tetrahedral
109.5°
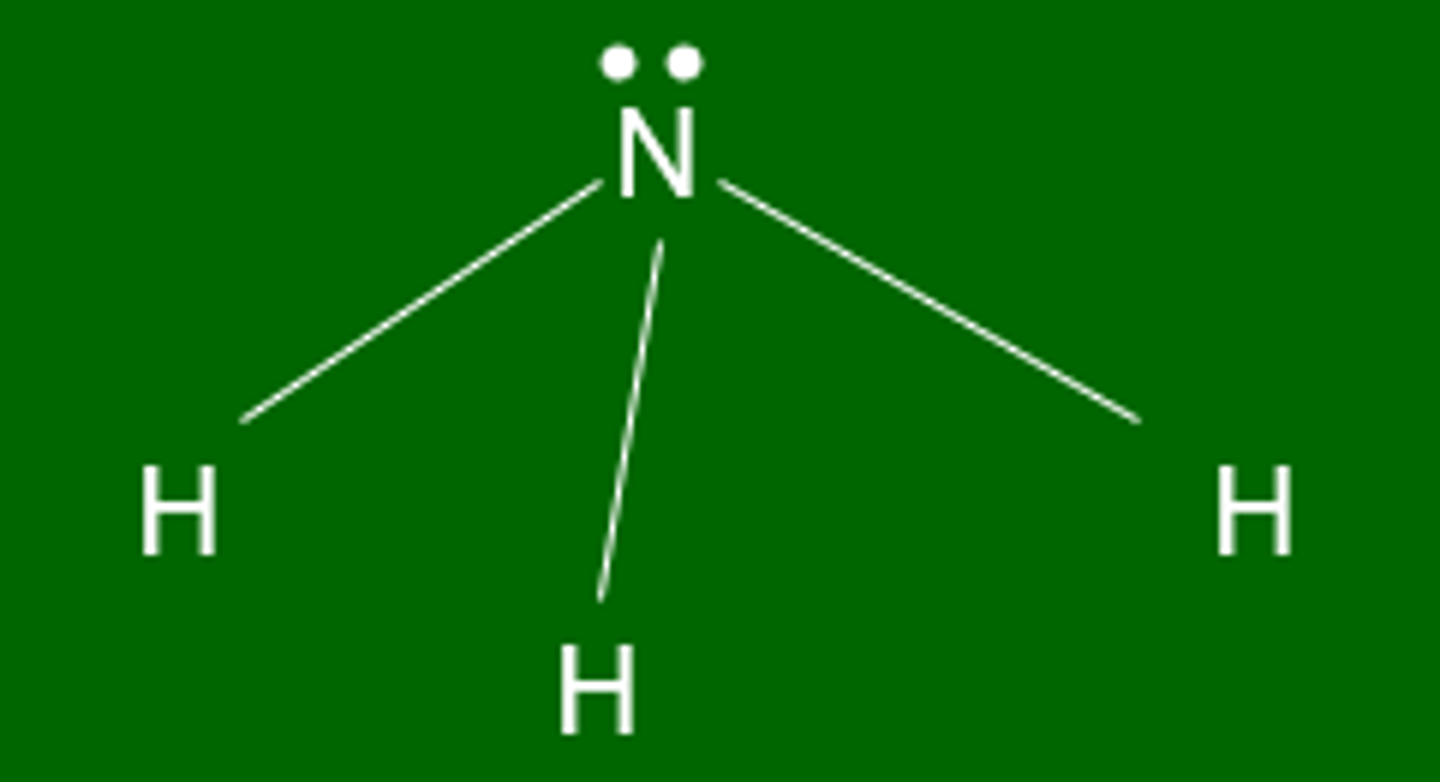
Shape of molecule: 4 electron pairs, 1 lone pair
1 lone pair
trigonal pyramidal
107°
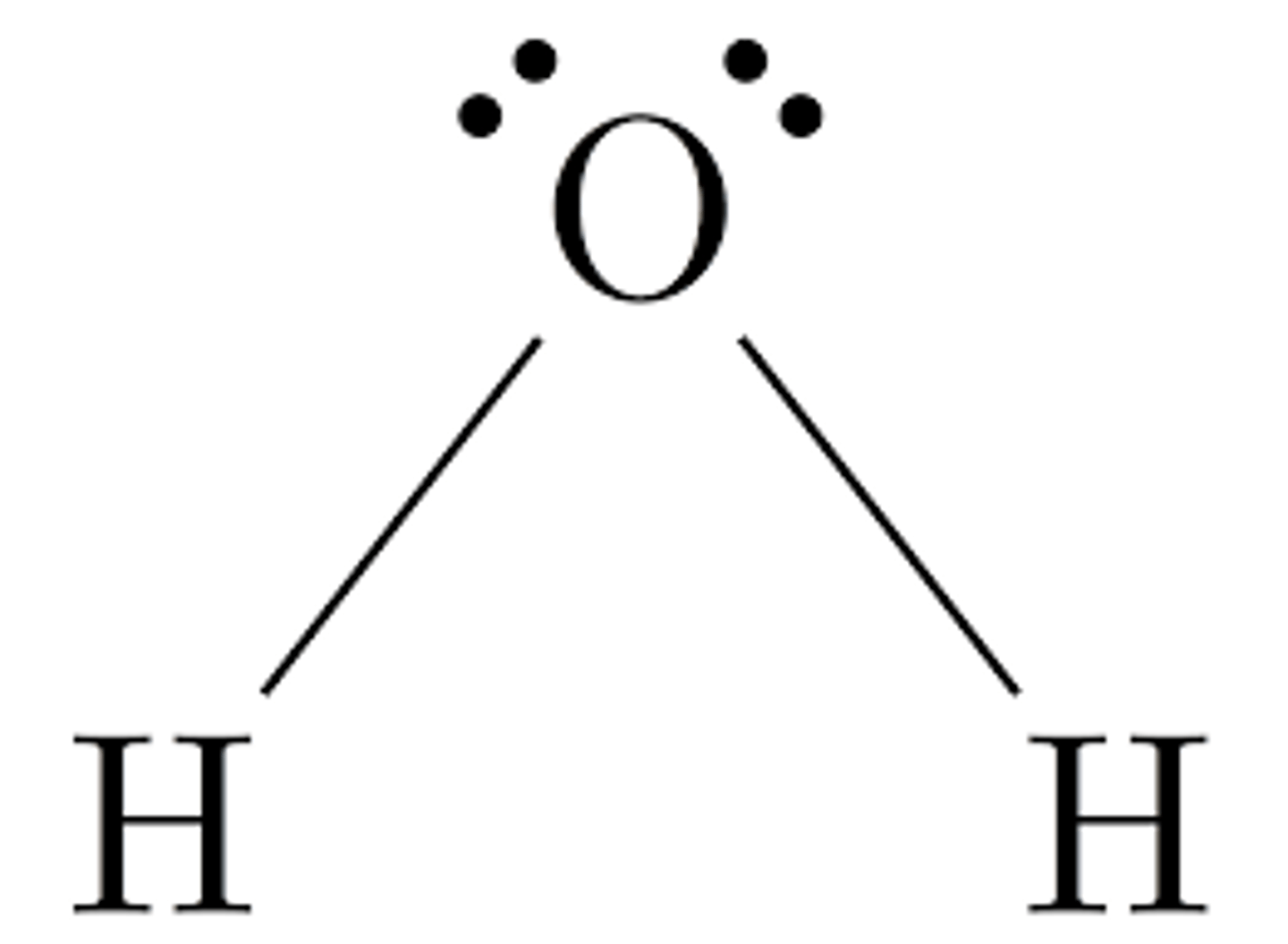
Shape of molecule: 4 electron pairs 2 lone pairs
2 lone pairs
bent
104.5°
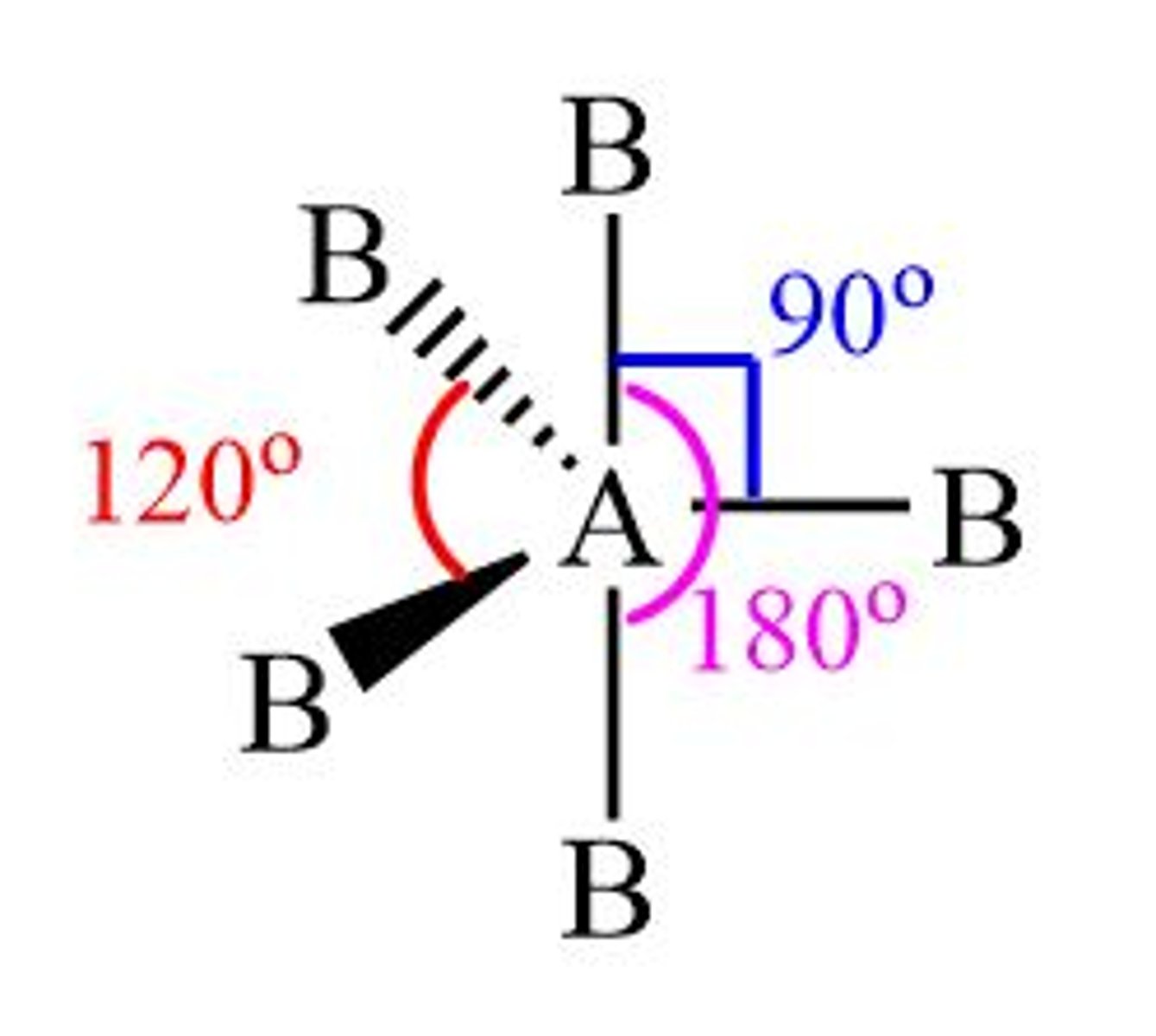
Shape of molecule: 5 electron pairs, no lone pairs
no lone pairs
trigonal bipyramidal
90°
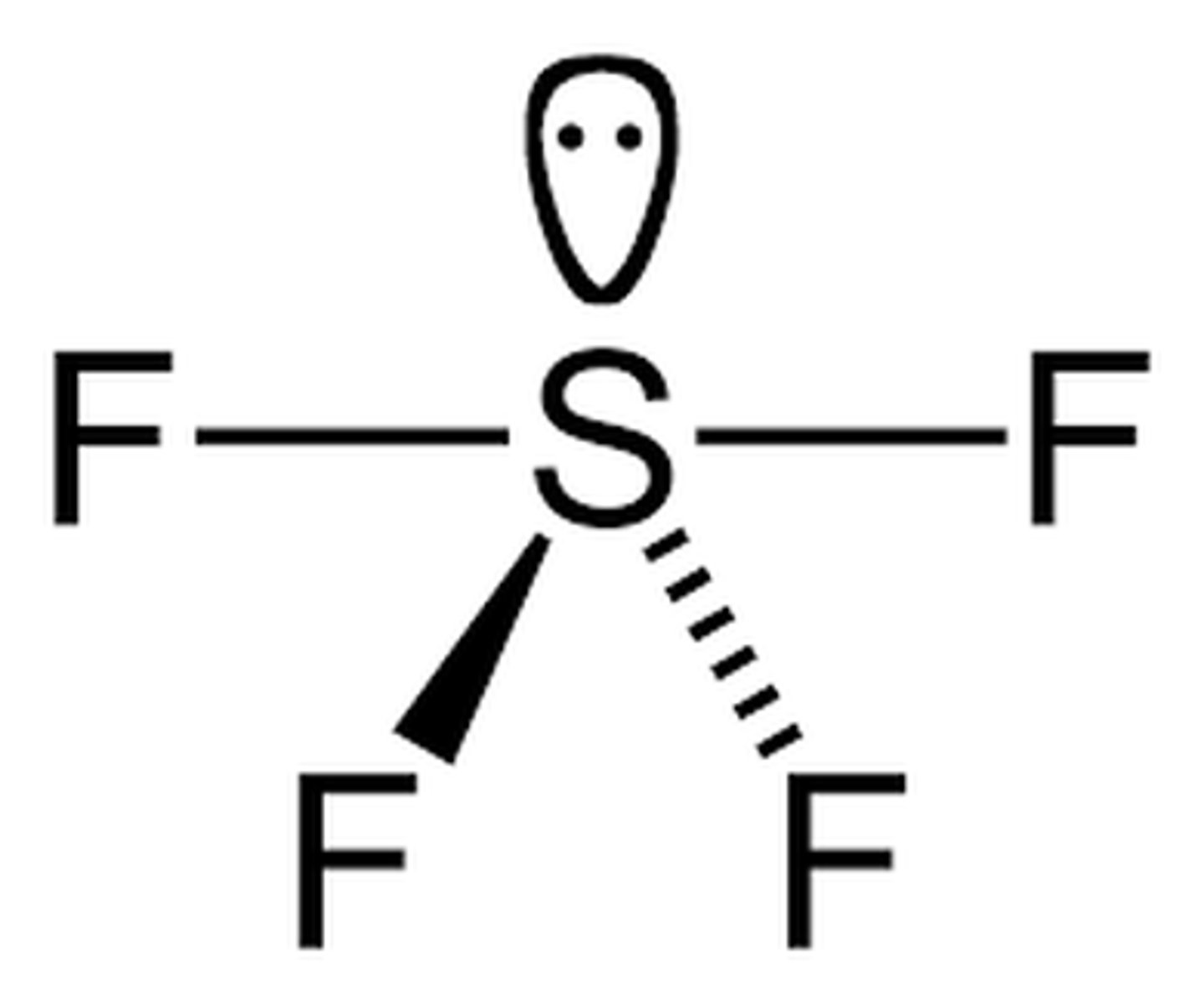
Shape of molecule: 5 electron pairs, 1 lone pair
1 lone pair
seesaw
87°
Shape of molecule: 5 electron pairs, 2 lone pair
2 lone pairs
T-shaped
88°
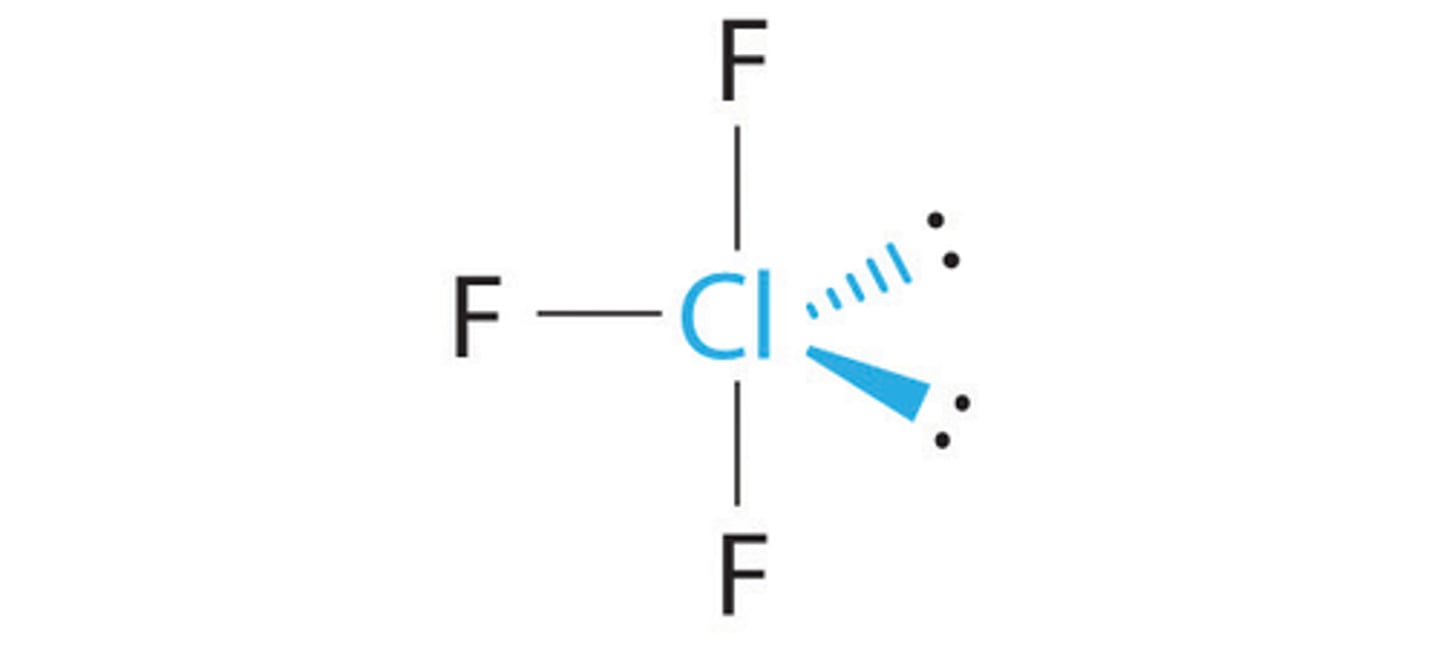
Shape of molecule: 6 electron pairs, no lone pairs
no lone pairs
octahedral
90°
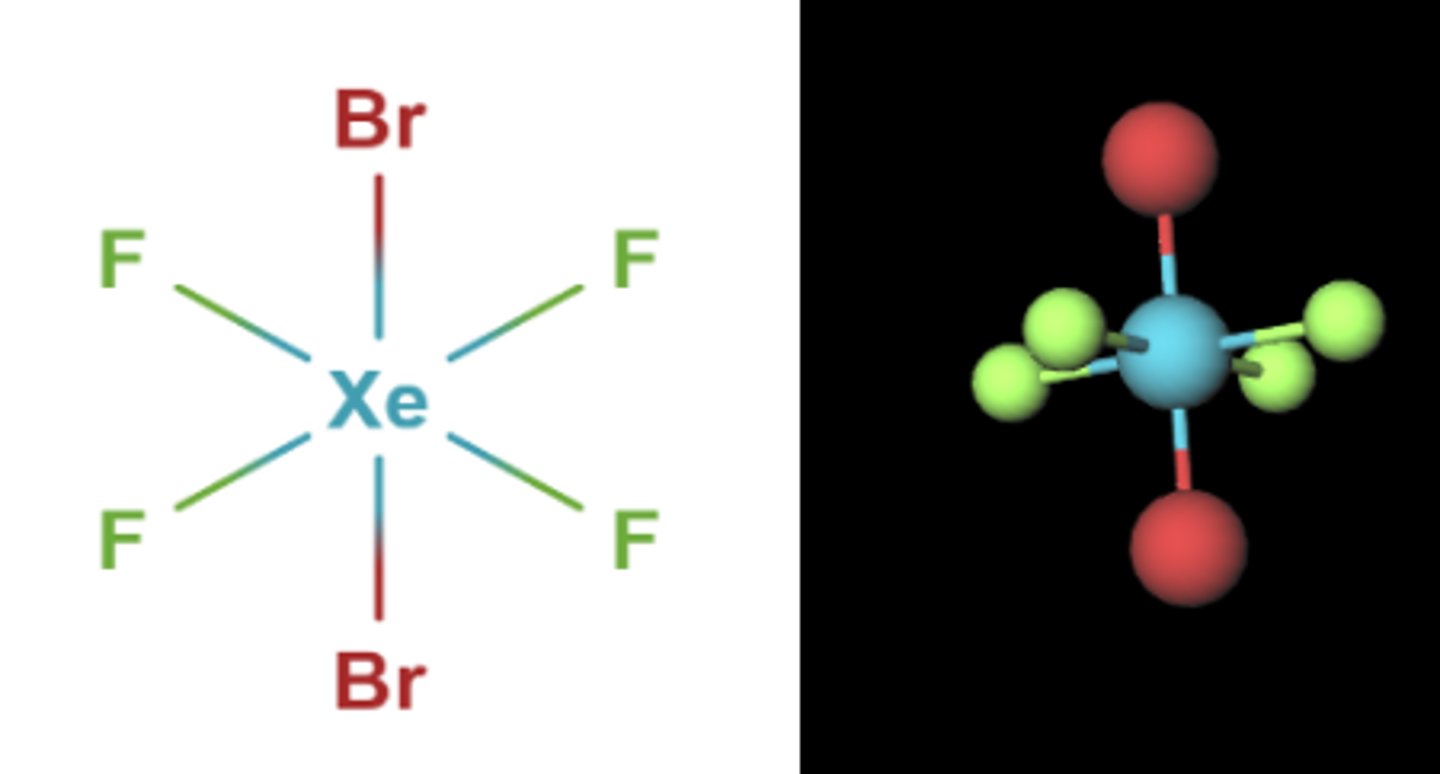
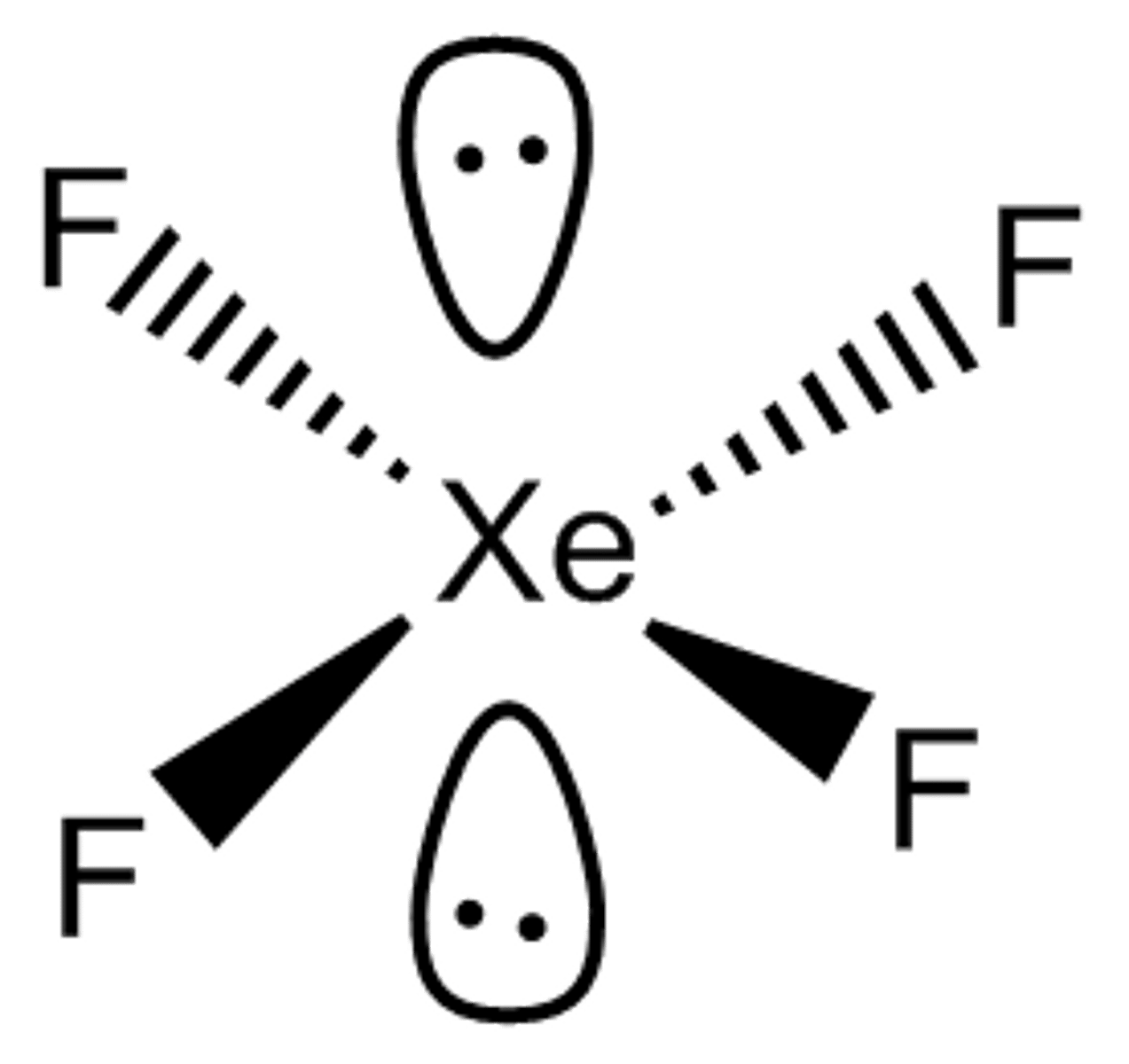
Shape of molecule: 6 electron pairs, 2 lone pairs
2 lone pairs
square planar
90°
Electronegativity
A measure of the ability of an atom in a chemical compound to attract electrons
What makes a Covalent Bond Polar
in a covalent bond between two atoms of different electronegativities
the bonding electrons will be pulled towards the more electronegative atom
> makes the bond polar
greater the difference in electronegativity, the more polar the bond
Which Covalent Bonds are Non-Polar
covalent bond between two atoms of the same element is polar since the atoms have equal electronegativities (eg H2)
some elements (like C and H) have similar electronegativities so bonds between them are non polar
Pauling Scale
the unit used to measure electronegativity