31. immune mediated diseases part I (type I & II hypersensitivities)
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
27 Terms
examples of chronic type I hypersensitivities
atopic dermatitis
asthma
what body regions are typically affected in atopic dermatitis?
feet, face, ventrum
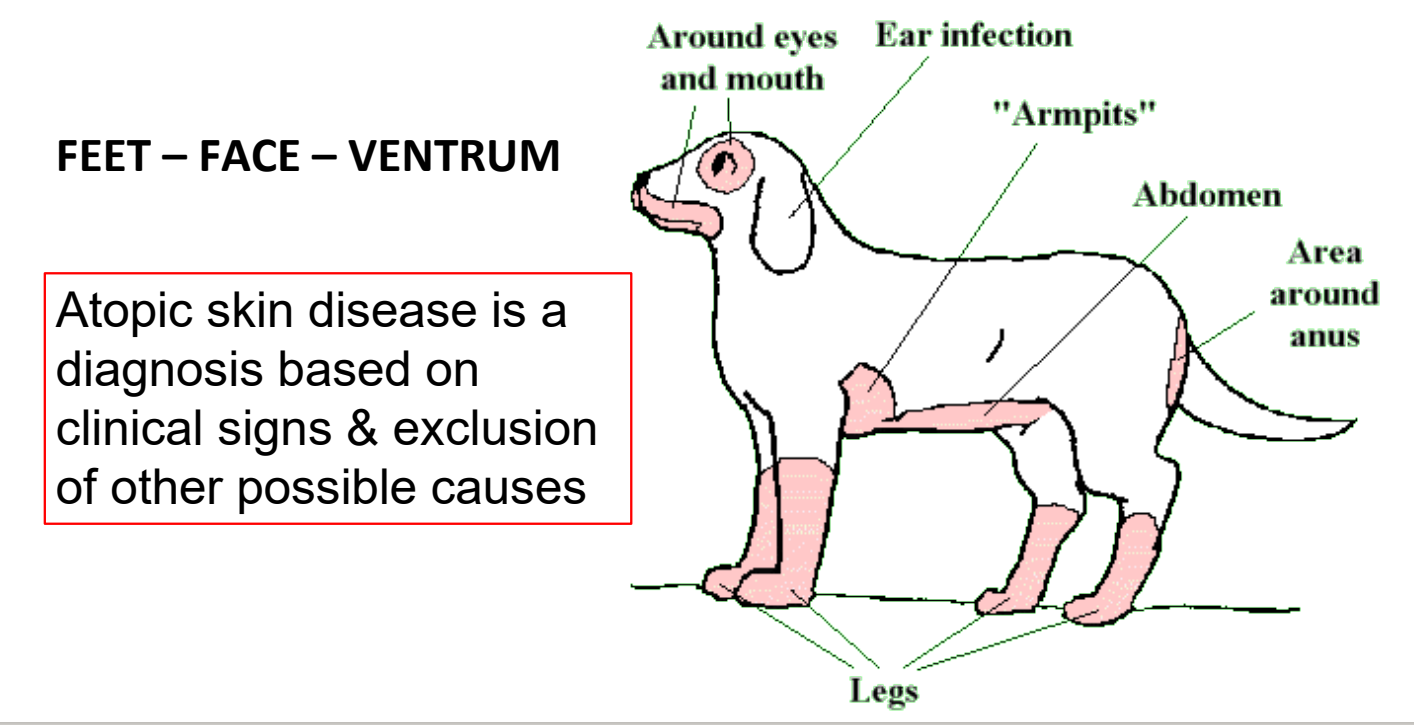
progression of atopic dermatitis
genetic predisposition for TH2 responses & IgE production
allergen is introduced & elicits response
secretion of TH2 cytokines → allergen-specific IgE production → IgE binds to mast cells/eosinophils → degranulation when IgE binds antigen
chronic phase: pro-inflammatory mediators attract other leukocytes into tissue
how is intradermal skin testing used in cases of atopic dermatitis?
NOT used to diagnose (normal dogs & cats can test positive)
used to figure out which antigens patient is sensitive to and formulate immunotherapy
treatment of atopic dermatitis
if possible, minimize exposure to allergen
treat any secondary infections (ex. pyoderma or otitis externa)
immunotherapy with “allergy shots” or “allergy drops”
designed to ↑ Tregs , ↓ TH2 & shift antibody production from IgE to IgG
can take several months to a year to see effect
glucocorticoids during crises
treatments to reduce/suppress pruritus
apoquel
cytopoint
feline asthma pathology
TH2 response & IgE production → mast cells
eosinophils are recruited to the airways during later stages by inflammatory mediators released by a variety of cells (lymphocytes, activated epithelium)
→ eosinophilia
treatment of feline asthma
medications → multiple routes, including orally & by aerosol
glucocorticoids (anti-inflammatory)
bronchodilators
environmental modulation: reduce exposure to allergens (dust, molds, pollens) & irritants (e.g. candles, air fresheners)
examples of type II hypersensitivities
immune-mediated hemolytic anemia
myasthenia gravis
pemphigus foliaceus
what signalment is most affected by immune-mediated hemolytic anemia? what is the prognosis?
one of the most common immune-mediated diseases in dogs
usually affects middle-aged female dogs
long-term prognosis is guarded
high mortality rate in dogs (~50%); mortality rate lower in cats
what are causes of immune-mediated hemolytic anemia?
primary (idiopathic) → more common in dogs
secondary → manifestation of underlying disease process
infectious (rickettsial organisms, heartworm, M. haemofelis)
neoplastic (leukemia, lymphoma)
drugs (cephalosporin & penicillin)
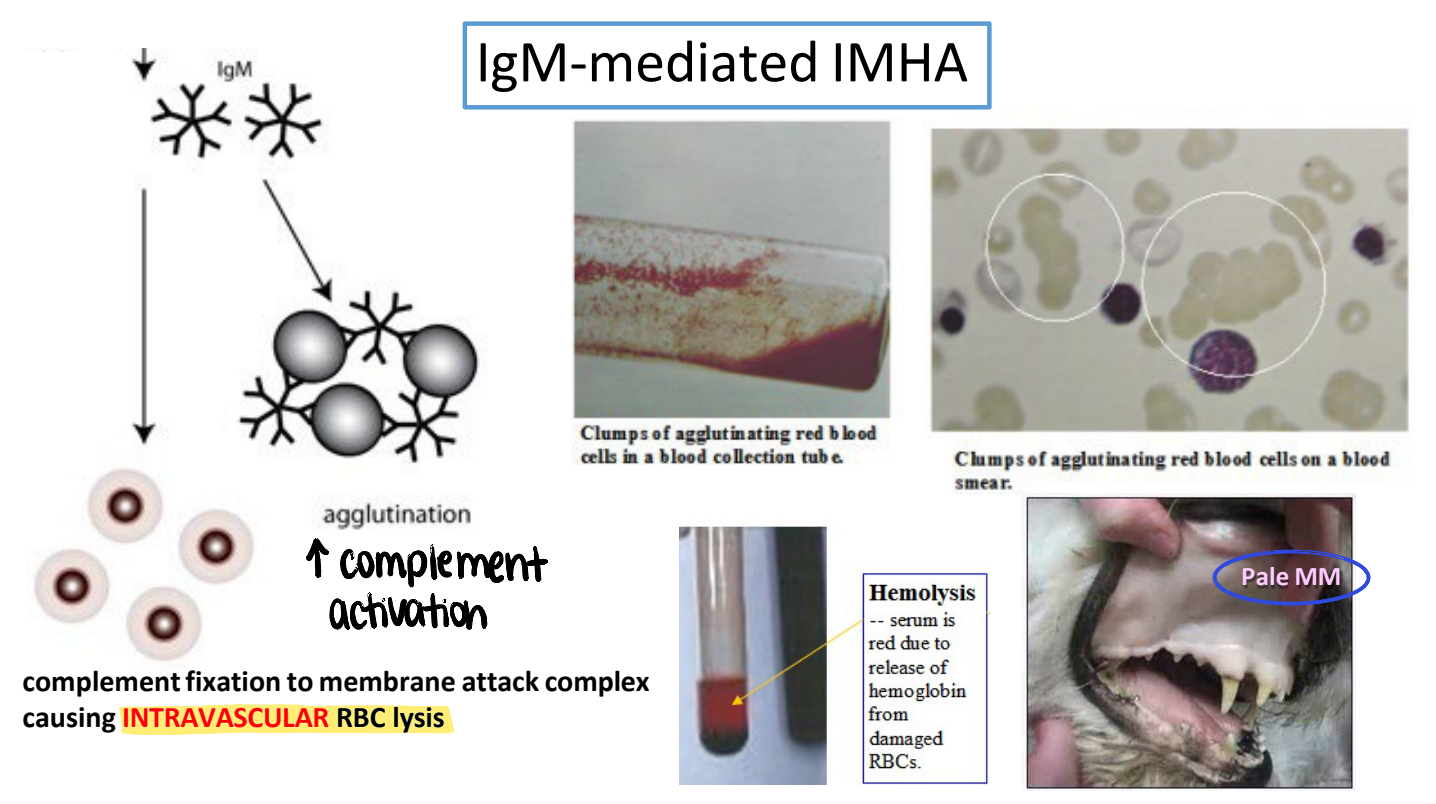
what is the typical appearance of mucous membranes in IgM-mediated IMHA? why?
pale MM
IgM (large pentamer) → ↑ agglutination → ↑ complement activation & membrane attack complex → intravascular RBC lysis
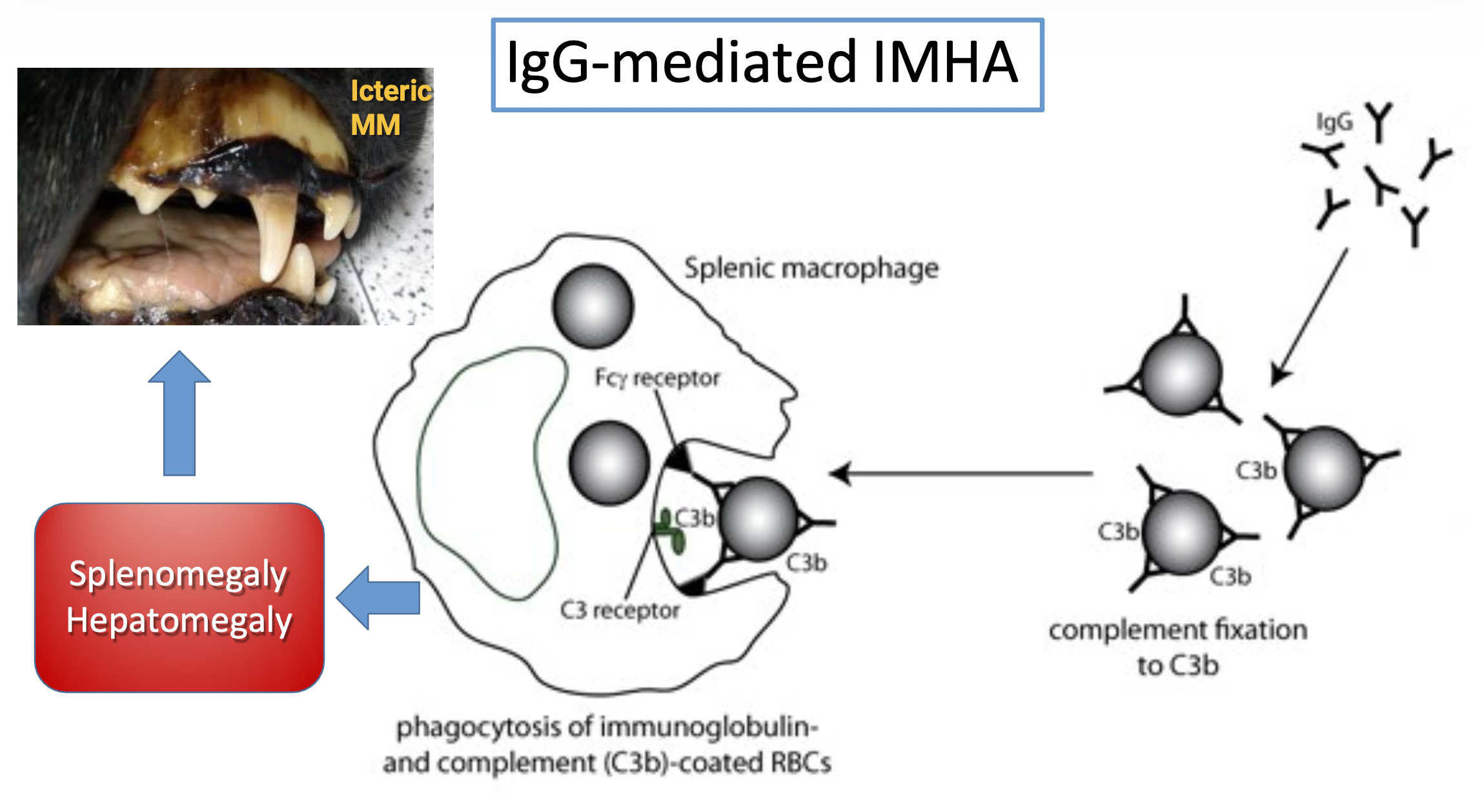
what is the typical appearance of mucous membranes in IgG-mediated IMHA and why? what are other clinical manifestations?
icteric MM
IgG → ↓ agglutination → phagocytosis by splenic macrophages → splenomegaly & hepatomegaly
high RBC breakdown → increased bilirubin
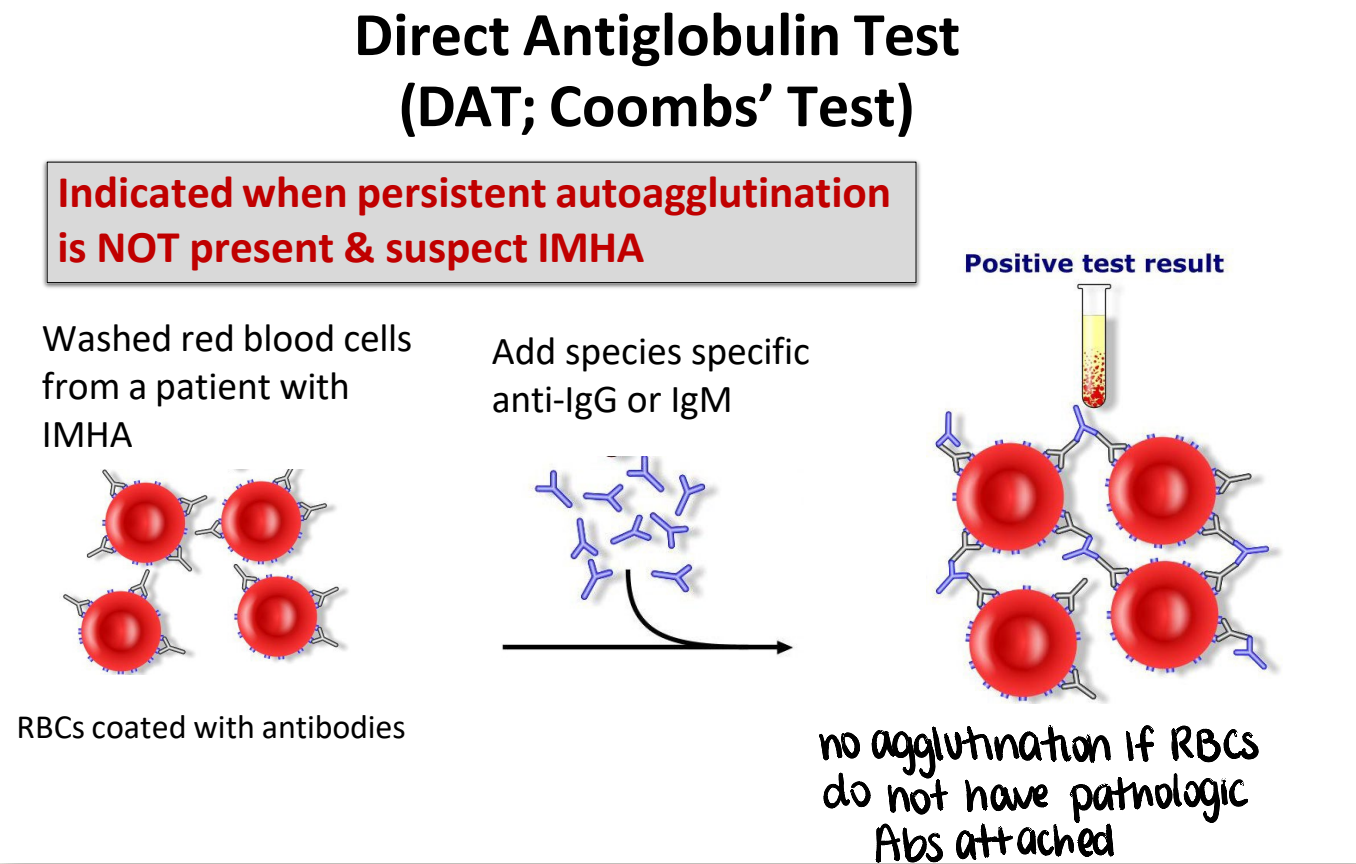
diagnostic findings consistent with IMHA
severe anemia
may be regenerative
spherocytes (dogs only, IgG-mediated)
ghost cells (IgM-mediated)
persistent autoagglutination of RBCs
positive coombs’ test (direct antiglobulin test)
bone marrow biopsy shows phagocytosis of erythroid precursors (if non-regenerative)
slide agglutination test
dilution of blood with saline will eliminate rouleaux
true agglutination will persist because antibodies to RBCs are response for clumping

direct antiglobulin test (coombs’ test)
washed RBCs from patient with IMHA → add species specific anti-IgG or IgM → agglutination
indicated when persistent autoagglutination is NOT present & suspect IMHA
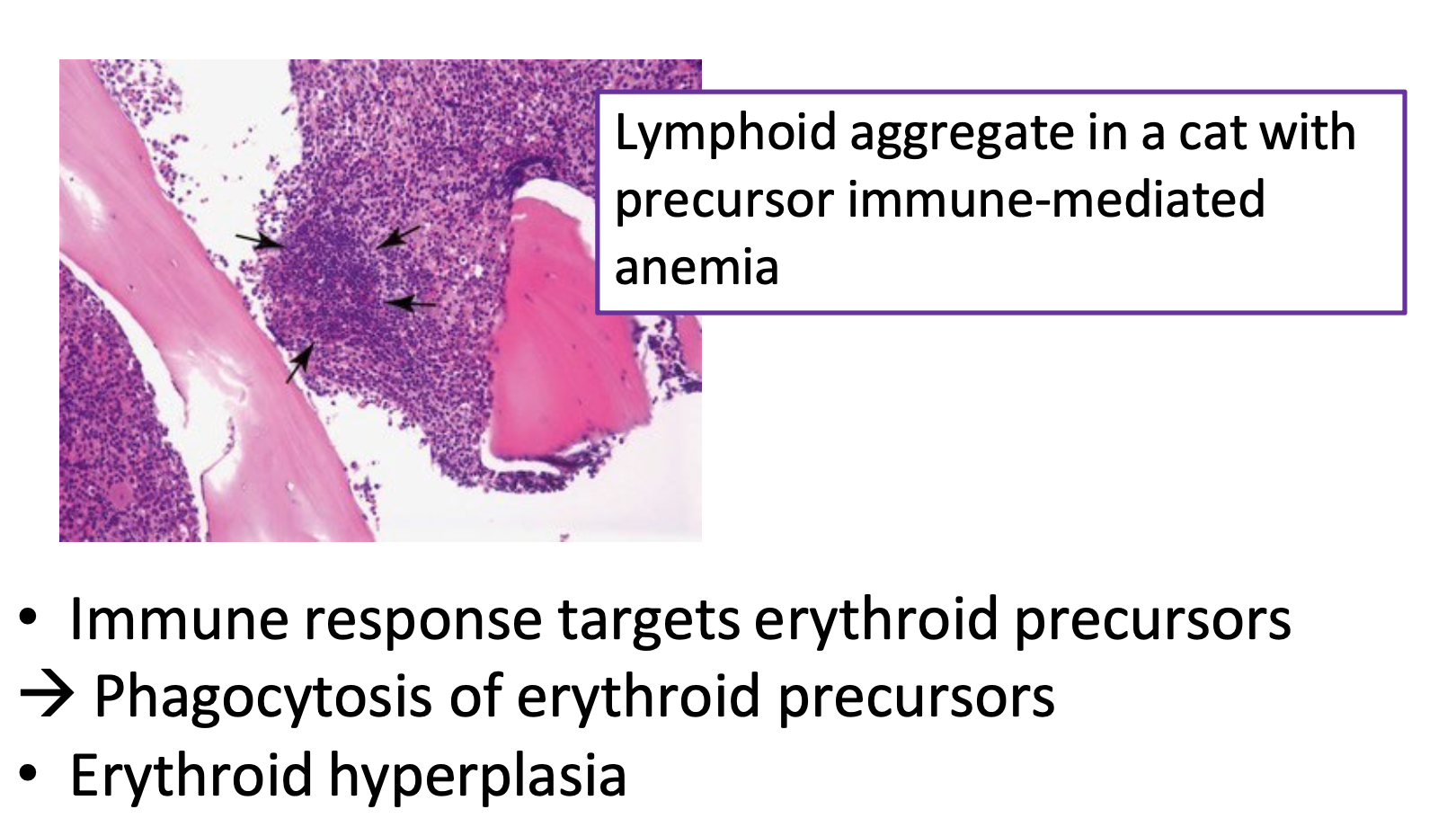
bone marrow aspirate (non-regenerative IMHA)
immune response targets erythroid precursors → phagocytosis of erythroid precursors
immature RBCs destroyed as they are produced
erythroid hyperplasia
primary IMHA treatment
glucocorticoids
inhibits Fc-receptor-mediated phagocytosis of RBCs by macrophages
inhibits complement activation
decreases production of pro-inflammatory cytokines
initial doses high to induce remission, then taper down to maintenance level
what are second-line IMHA treatments? when should they be used?
immunosuppressive agents
azathioprine → dogs
chlorambucil → cats
added if:
severe disease
intravascular hemolysis or transfusion dependency
lack of response to glucocorticoid therapy alone
concurrent thrombocytopenia
significant glucocorticoid side effects
acquired myasthenia gravis pathology
antibodies directed against acetylcholine receptors on muscle cells → receptor degradation or blockage of neuromuscular transmission
causes muscle weakness; megaesophagus
what signalment is affected by myasthenia gravis?
bimodal distribution in dogs
2-4 years & 9-13 years
rare in cats
signs of muscle weakness (myasthenia gravis)
focal with selective involvement of esophageal, pharyngeal, and facial muscles
diffuse with signs of generalized muscle weakness
acute fulminating — rapid onset of appendicular muscle weakness, megaesophagus, & collapse
myasthenia gravis diagnosis
detection of antibodies to acetylcholine receptor (gold standard)
physical exam & radiographs (megaesophagus ± chest mass [thymoma])
tensilon response test
inhibits acetylcholinesterase → acetylcholine can remain longer in the synaptic cleft & stimulate any remaining receptors
currently not being manufactured
electromyogram
decreasing amplitude
![<ul><li><p><strong>detection of antibodies to acetylcholine receptor (gold standard)</strong></p></li><li><p>physical exam & radiographs (megaesophagus ± chest mass [thymoma])</p></li><li><p>tensilon response test</p><ul><li><p>inhibits acetylcholinesterase → acetylcholine can remain longer in the synaptic cleft & stimulate any remaining receptors</p></li><li><p>currently not being manufactured</p></li></ul></li><li><p>electromyogram</p><ul><li><p>decreasing amplitude</p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/627f8007-d740-41c9-812c-1eeecd3711ac.png)
myasthenia gravis treatment
first line — acetylcholinesterase inhibitors
immune suppression — controversial
(megaesophagus — risk of aspiration pneumonia)
use only if necessary
plasmapheresis
helpful in severe disease or refractory cases
what signalments/species does pemphigus foliaceus affect?
dogs, cats, horses
most common immune-mediated skin disease
middle-aged to older animals; some breed dispositions
pemphigus foliaceus clinical signs/distribution of lesions
superficial pustules (subcorneal, vesicopustules) — fragile!
lesions break open → crusting, exfoliation, erythema, erosions
lesions often GENERALIZED
face - bridge of nose & muzzle
ears
groin
footpads

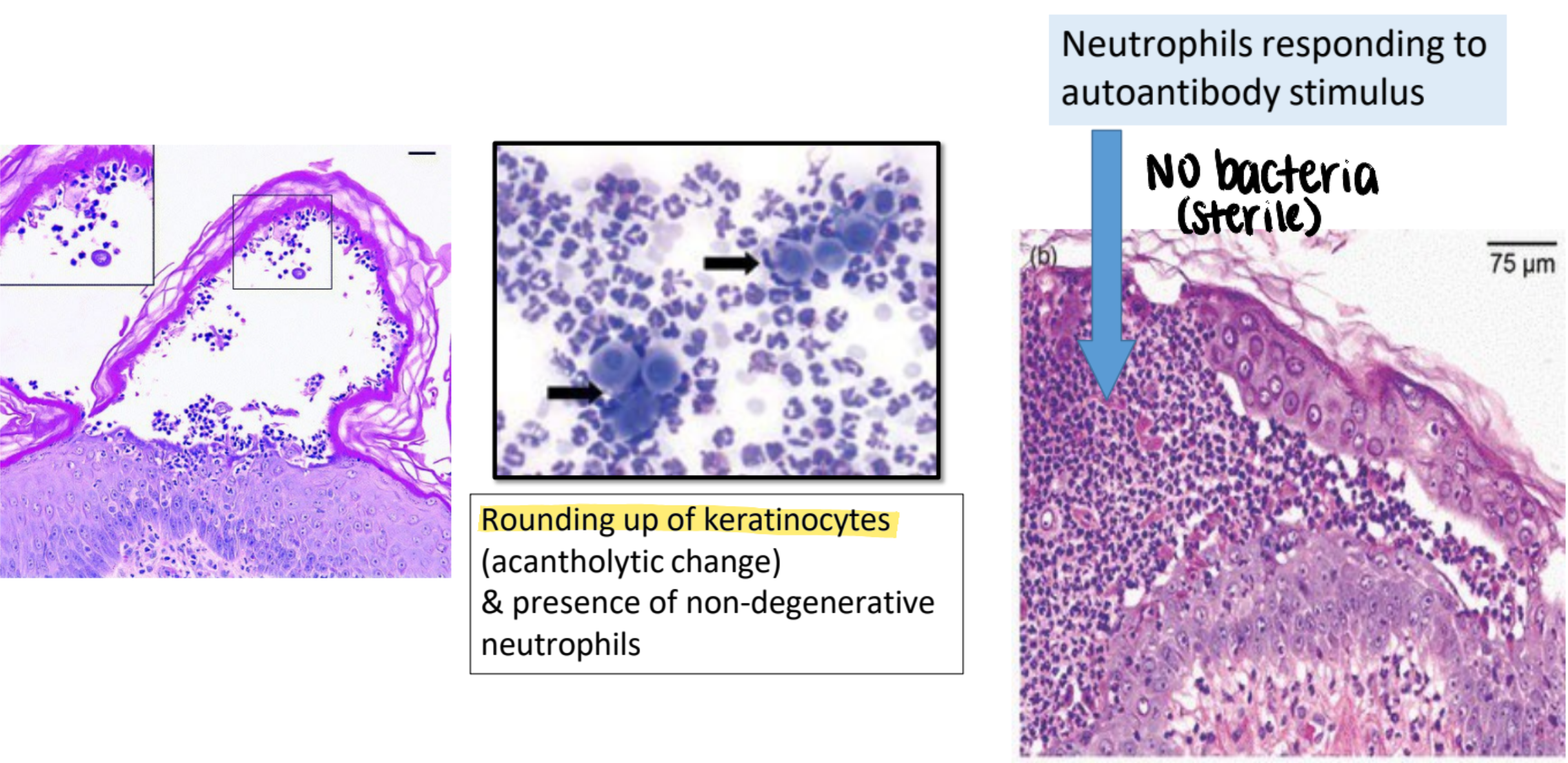
pemphigus foliaceus pathology
autoantibodies directed against desmosomes (extracellular cement protein of epidermis; anchors epidermal cells)
neutrophils respond to autoantibody stimulus but no bacteria
rounding up of keratinocytes (acantholytic change) & presence of non-degenerative neutrophils
pemphigus foliaceus treatment
glucocorticoids most commonly used & are the first choice
combination treatment — azathioprine (dogs) or chlorambucil
treat any secondary bacterial infections that may be present
avoid medications that may predispose to immune attacks
drugs that contain carbon-bonded sulfhydryl (-SH) group — beta-lactam antibiotics & trimethoprim-sulfa drugs
avoid sunlight — UV radiation exacerbates the disease