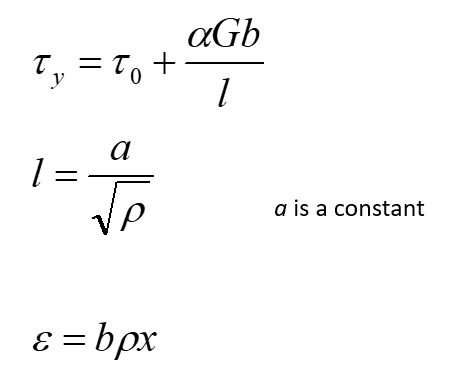L7- Point defects + dislocation density
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
Examples of point defects intrinsic
Intrinsic defects:
Vacancies
self-interstitials
What are the ranges of defects and changes in microstructure plastic deformation produces?
point defects
changes in grain shape, orientation and/or distribution
increase in dislocation density
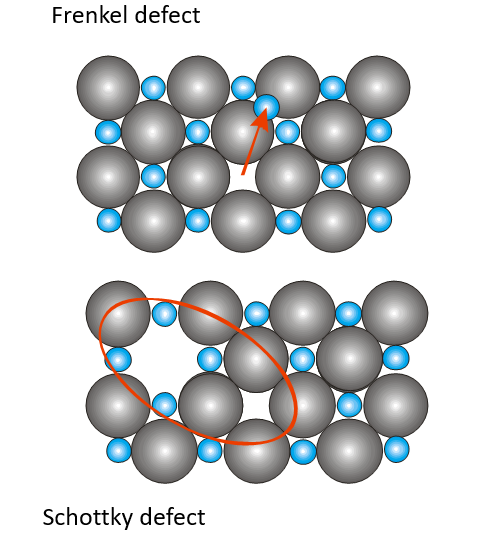
Point defects in ionic crystals:
Frenkel defect
Schottky defect
occur in pairs in ionic materials to maintain charge neutrality
Examples of point defects extrinsic
solid solution atoms
substitution
interstitial
*point defects are associated with strain field
Equation for equilibrium concentration of vacancies:
N = no. atoms
neq = no. vacancies at equilibrium
ΔH f= Energy of formation of 1 vacancy site
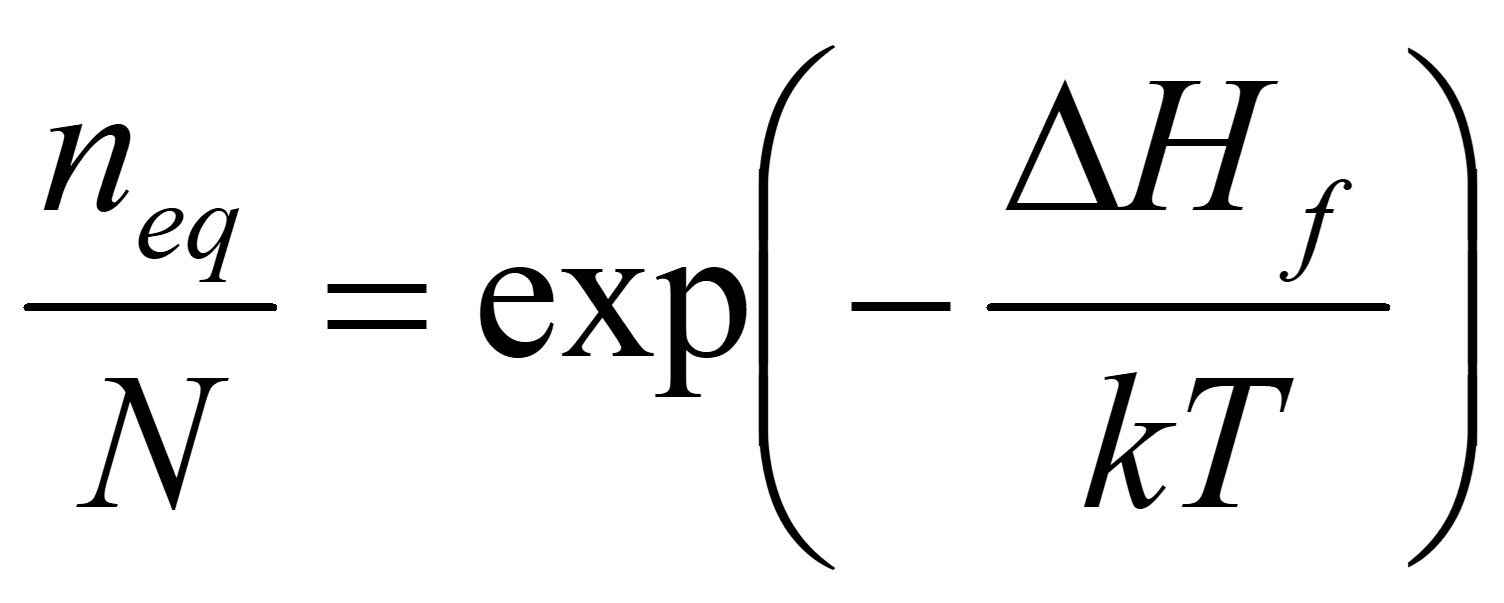
Where can point defects be absorbed ?
grain boundaries
surfaces
dislocations
Point defect properties:
mobile, particularly interstitials
can also form aggregates in the case of vacancies these would result in voids
Properties of substitutional solutes:
•low diffusivity, solute atoms fixed
•atoms randomly distributed
•dislocations sample solute atoms
•resistance to flow function of atom size mismatch + solute concentration
Properties of interstitial solutes:
•high diffusivity, solute atoms concentrate
•segregate to dislocations
•low concentration of solute has disproportionate effect in pinning dislocations
Effect of impurities on lattice :
generates σ by distorting lattice
σ creates barrier to dislocation motion
What does strengthening due to substitutional solutes depend on?
size difference between solute + matrix atoms
solute concentration
Substitutional solutes formulae
a – lattice parameter
c – concentration
θ– lattice mismatch
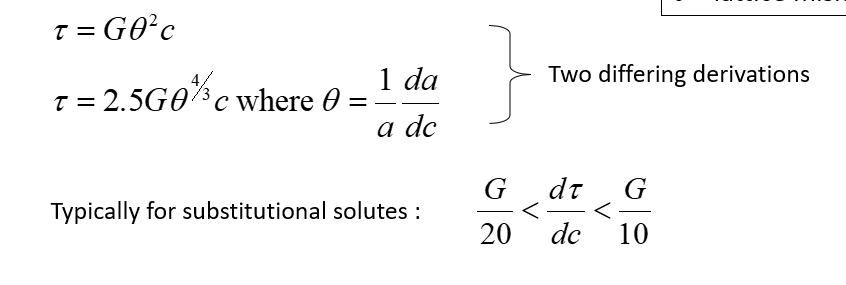
What does strengthening due to interstitial solutes depend on?
tetragonal stress fields that inhibit the mobility of both edge and screw dislocations
Segregation of solutes to dislocations

Why do interstitial solutes have high diffusivity
Interstitial solutes, such as C, O, N, and H, have high diffusivity due to their small atomic mass and ability to occupy small gaps in the lattice.
solutes strengthen materials by segregating to dislocations, reducing energy by filling space in the tensile region, but needing ↑ stress to free the dislocation from the solute/ forcing it to diffuse with the solute atom.
Disadv of work hardening as a strengthening mechanism:
loss in ductility
necking + loss of ability to resist cracking
How to tell if an interaction is favourable ?
If dislocation burgers vector, b decreases
⇒ decrease in E = ½ Gb2
so favourable (Frank’s Rule)
Why do most materials work harden?
1.Dislocations are bound to encounter each other
2.These encounters produce sessile debris
3.This debris makes deformation harder
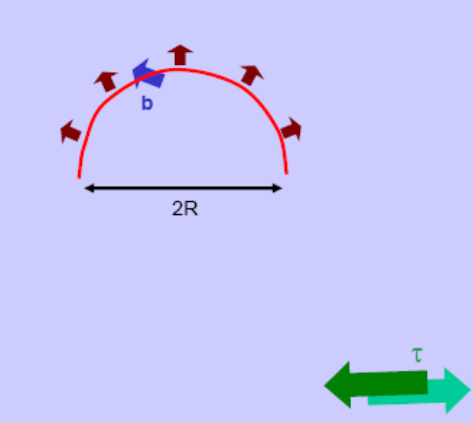
Stress to operate Frank Reed source
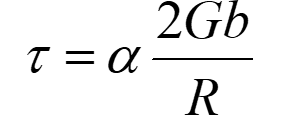
Force between screw dislocations
l = distance between 2 dislocations
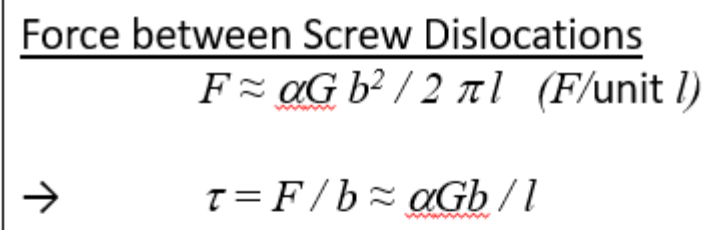
Taylor’s model of dislocation hardening assumptions:
yield strength increases with increasing density of obstacles
density of obstacles increases with increasing dislocation density
dislocation density increases with plastic strain
Taylor’s model equation:
x = glide distance / distance moved by dislocations
ρ = dislocation density