Didem Ahmed PBL
1/86
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
87 Terms
List causes of breathlessness in children
Asthma: This is one of the most common causes of breathlessness in children.
Pneumonia:
Bronchiolitis:
Croup:
Other Causes
Foreign object inhalation:
Anxiety
Heart conditions:
Allergic Reactions
Anaphylaxis
Hay fever:
A Perfect Breath Can Fuel All Happy Adventures
Describe the basic structure of DNA
DNA is composed of two strands coiled around each other in a double helix shape. The key components of DNA structure include:
Nucleotides: The basic building blocks of DNA, consisting of:
A sugar group (deoxyribose)
A phosphate group
A nitrogenous base
Backbone: The sugar and phosphate groups form the "backbone" of each DNA strand.
List the nitrogenous bases in DNA
Nitrogenous Bases: There are four types:
Adenine (A)
Thymine (T)
Guanine (G)
Cytosine (C)
Describe base pairing and directionality in DNA
Base Pairing: The two DNA strands are held together by hydrogen bonds between complementary base pairs:
Adenine (A) pairs with Thymine (T)
Guanine (G) pairs with Cytosine (C)
Directionality: The two DNA strands run in opposite directions (antiparallel) to each other.
What is a gene
A gene is a section of DNA that contains the instructions for making a specific protein.
Describe the structure of genes
Coding Regions (Exons): These contain the actual instructions for building proteins.
Non-coding Regions (Introns): These are sections within genes that are removed before protein synthesis.
Regulatory Regions: These control when and how much of a gene is expressed, including:
Promoter: A sequence that initiates gene transcription
Enhancers: Sequences that can increase gene expression
What is a genetic mutation
Mutations are changes in the DNA sequence that can lead to genetic variation.
List the types of genetic mutations
Point Mutations: Changes in a single nucleotide base
Substitution: One base is replaced by another
Insertion: An extra base is added
Deletion: A base is removed
Chromosomal Mutations: Large-scale changes affecting chromosome structure or number
What are the effects of mutations
Silent mutations: No change in amino acid sequence
Missense mutations: Change in amino acid sequence
Nonsense mutations: Premature stop codon, truncating the protein
Frameshift mutations: Alter the reading frame of the genetic code
What is a silent mutation
No change in amino acid sequence
what is a missense mutation
A single nucleotide change results in the substitution of one amino acid for another in the protein produced.
This can affect the protein's function, potentially leading to various effects on the organism, depending on the role of the altered amino acid.
What is a nonsense mutation
A single nucleotide change results in a premature stop codon in the coding sequence of a gene.
This leads to the production of a truncated protein, which is usually nonfunctional, potentially causing various genetic disorders or diseases.
What is a frameshift mutation
A genetic mutation caused by the insertion or deletion of nucleotides in a DNA sequence that shifts the reading frame of the genetic code.
This alteration can lead to changes in the amino acid sequence of a protein, potentially resulting in a nonfunctional protein or a completely different protein.
Frameshift mutations can have significant effects on an organism's phenotype.
What is transciption of DNA
Transcription is the process of copying DNA into RNA. The main steps are:
Initiation
Elongation
Termination
Processing
Describe in detail the process of transciption
Initiation:
RNA polymerase binds to the promoter region of a gene
The DNA double helix unwinds at the transcription start site
Elongation:
RNA polymerase moves along the template DNA strand in the 3' to 5' direction
Complementary RNA nucleotides are added to the growing RNA chain in the 5' to 3' direction
The RNA transcript is synthesized using base pairing rules (A pairs with U, C with G)
Termination:
RNA polymerase reaches a termination sequence
The newly synthesized RNA transcript is released
The DNA double helix reforms
Processing (in eukaryotes):
Addition of 5' cap and 3' poly-A tail
Splicing to remove introns and join exons
The result is a mature messenger RNA (mRNA) molecule.
What occurs as part of initiation in DNA transcription
RNA polymerase binds to the promoter region of a gene
The DNA double helix unwinds at the transcription start site
What occurs as part of elongation in DNA transcription
RNA polymerase moves along the template DNA strand in the 3' to 5' direction
Complementary RNA nucleotides are added to the growing RNA chain in the 5' to 3' direction
The RNA transcript is synthesized using base pairing rules (A pairs with U, C with G)
What occurs as part of termination in DNA transcription
RNA polymerase reaches a termination sequence
The newly synthesized RNA transcript is released
The DNA double helix reforms
What occurs as part of processing in DNA transcription
Addition of 5' cap and 3' poly-A tail
Splicing to remove introns and join exons
The result is a mature messenger RNA (mRNA) molecule.
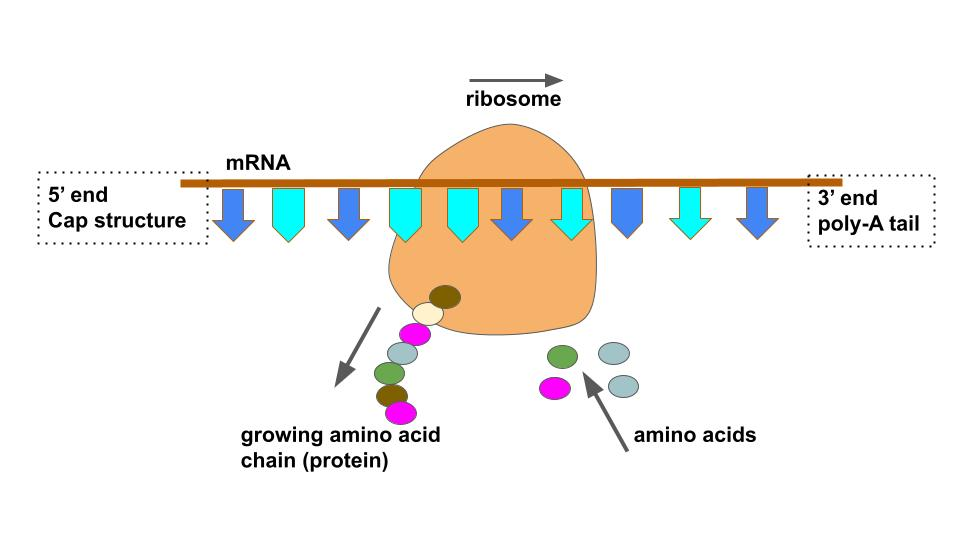
Describe processing as a part of DNA transcription
During DNA transcription, processing refers to the modifications that pre-mRNA undergoes before becoming mature mRNA. This includes:
Capping: Addition of a 5' cap to the beginning of the mRNA for stability and recognition.
Polyadenylation: Addition of a poly-A tail at the 3' end, enhancing stability and export from the nucleus.
Splicing: Removal of introns (non-coding regions) and joining of exons (coding regions) to form a continuous coding sequence.
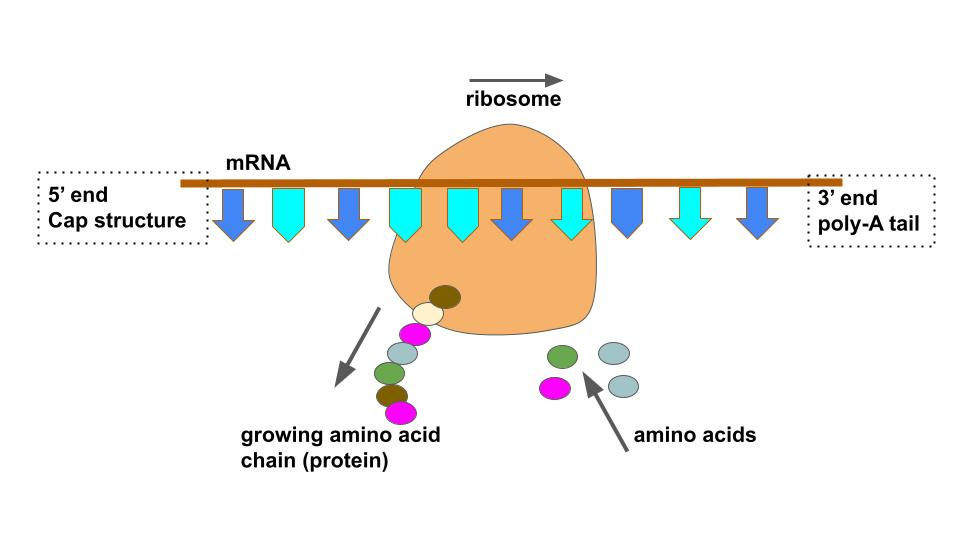
What is DNA Translation
Translation is the process of synthesizing a protein using the mRNA as a template. The main steps are:
Initiation:
Elongation:
Termination:
Post-translational modifications:
Describe in detail the process of DNA translation
Initiation:
The small ribosomal subunit binds to the mRNA at the start codon (AUG)
The large ribosomal subunit joins to form the complete ribosome
Elongation:
tRNA molecules bring amino acids to the ribosome
The ribosome reads the mRNA codons in sets of three nucleotides
Amino acids are joined together by peptide bonds to form the growing polypeptide chain
Termination:
The ribosome reaches a stop codon (UAA, UAG, or UGA)
The completed polypeptide is released
The ribosome dissociates from the mRNA
Post-translational modifications:
The polypeptide may undergo further modifications (e.g. folding, glycosylation)
What occurs as part of initiation in DNA translation
The small ribosomal subunit binds to the mRNA at the start codon (AUG)
The large ribosomal subunit joins to form the complete ribosome
What occurs as part of elongation in DNA translation
tRNA molecules bring amino acids to the ribosome
The ribosome reads the mRNA codons in sets of three nucleotides
Amino acids are joined together by peptide bonds to form the growing polypeptide chain
What occurs as part of termination in DNA translation
The ribosome reaches a stop codon (UAA, UAG, or UGA)
The completed polypeptide is released
The ribosome dissociates from the mRNA
What occurs as part of Post-translational modifications in DNA translation
The polypeptide may undergo further modifications (e.g. folding, glycosylation)
What are the common symptoms of anaemia
Fat People Should Definitely Run Hard, Coldly Breathing Greatly
F - Fatigue
P - Paleness
S - Shortness of breath
D - Dizziness or lightheadedness
R - Rapid heartbeat (palpitations)
H - Headaches
C - Cold hands and feet
B - Brittle nails
G - Growth problems (in children)
List the causes of microcytic anaemia MCV<80
Iron deficiency anaemia: This is the most common cause of microcytic anaemia.
Thalassemia: An inherited blood disorder affecting haemoglobin production.
Anaemia of chronic disease: e.g. kidney disease, certain cancers, and inflammatory diseases
List the causes of normocytic anaemia MCV 80-100
Acute blood loss
Hemolytic disorders:
Inherited conditions like sickle cell disease, thalassemias
Acquired conditions like autoimmune hemolytic anemia
Bone marrow disorders:
Aplastic anemia
Myelodysplastic syndromes
Pure red cell aplasia
Chronic kidney disease
A Happy Bear Can
List the causes of macrocytic anaemia
Vitamin B12 deficiency: This is one of the most common causes.
Folate (vitamin B9) deficiency: Another very common cause, especially during pregnancy.
Alcoholism: Chronic alcohol use can lead to macrocytic anemia.
Liver disease: Various liver conditions can contribute to this type of anemia.
Hypothyroidism: An underactive thyroid can cause macrocytic anemia.
Big Fish Always Live Happy
Where do erythrocytes originate
Produced in bone marrow through erythropoiesis
Develop from hematopoietic stem cells
Describe the structure of erythrocytes
Biconcave disk shape, 7-8 μm in diameter
No nucleus or organelles
Flexible membrane composed of proteins and lipids
Cytoplasm filled with hemoglobin
What is the function of erythrocytes
Transport oxygen from lungs to tissues
Transport carbon dioxide from tissues to lungs
Regulate blood pH
Where are WBCs produced
Produced in bone marrow
Develop from hematopoietic stem cells
Describe the structure of WBCs
Vary in size and shape depending on type
Contain nucleus and organelles
What are the types of WBCs
Neutrophils:
Lymphocytes:
Monocytes:
Eosinophils:
Basophils:
What is the function of neutrophils
Phagocytosis of bacteria and fungi
What is the function of lymphocytes
Produce antibodies and regulate immune response
What is the function of monocytes
Phagocytosis and antigen presentation
What is the function of eosinophils
Defense against parasites and allergic reactions
What is the function of basophils
Release histamine in allergic reactions
What is the origin of platelets
Produced in bone marrow
Fragment from megakaryocyte
Describe the structure of platlets
Small, irregularly shaped cell fragments
No nucleus
Contain granules with clotting factors
Describe the function of platelets
Blood clotting and hemostasis
Release growth factors for wound healing
Describe the structure of primary proteins
Linear sequence of amino acids
Held together by covalent peptide bonds between amino acids
Describe the structure of secondary proteins
Regular folding patterns within sections of the polypeptide chain
Main types: alpha-helices and beta-pleated sheets
Stabilized primarily by hydrogen bonds between the main-chain peptide groups
Alpha-helix: coiled structure with hydrogen bonds between every fourth amino acid
Beta-pleated sheet: extended zig-zag structure with hydrogen bonds between adjacent strands
Describe the structure of tertiary proteins
Overall three-dimensional shape of a single polypeptide chain
Stabilized by various interactions between amino acid side chains:
Hydrophobic interactions: major driving force for folding, involving non-polar side chains
Hydrogen bonds: between polar side chains
Ionic bonds: between oppositely charged side chains
Disulfide bridges: covalent bonds between cysteine residues
Van der Waals forces: weak interactions between nearby atoms
Describe the structure of quaternary proteins
Arrangement of multiple polypeptide chains (subunits) in a single functional protein
Stabilized by the same types of interactions as tertiary structure:
Non-covalent interactions (hydrophobic, hydrogen bonds, ionic bonds)
Sometimes disulfide bridges between subunits
Describe the structure of haemoglobin
Quaternary structure:
Tetramer composed of four subunits: two α and two β chains
Each subunit contains a heme group
Haeme group:
Iron-containing molecule of protoporphyrin-IX
Allows binding of oxygen and other gases
Conformational states:
T state (tense): low oxygen affinity, deoxygenated form
R state (relaxed): high oxygen affinity, oxygenated form
Describe the function of haemoglobin
Primary function: Oxygen transport
Binds oxygen in lungs and releases it in tissues
Increases oxygen-carrying capacity of blood by ~50-100 times
Secondary functions:
CO2 transport (10-20% of total CO2)
pH regulation
Nitric oxide metabolism
Cooperative binding:
Sigmoid oxygen dissociation curve
Allows efficient loading and unloading of oxygen
What are the adaptions of RBCs for oxygen delivery
High surface area to volume ratio for gas exchange
Flexibility to pass through small capillaries
Ability to deform without rupturing
Contain enzymes to maintain hemoglobin in reduced state
List the clinical features of Sickle cell anamia
Anaemia: Fatigue, shortness of breath, delayed growth and development in children
Painful episodes (vaso-occlusive crises): Acute pain in various parts of the body
Increased risk of infections
Jaundice and yellowing of the eyes
Hand-foot syndrome in infants (painful swelling of hands and feet)
Stroke risk, especially in children
Acute chest syndrome: Chest pain, fever, difficulty breathing
Pulmonary hypertension
Organ damage (kidneys, liver, spleen, brain)
Priapism in males
Leg ulcers
Vision problems, including potential blindness
APPAL JOSH VIP
List the pathological features of sickle cell anaemia
Abnormal hemoglobin S production
Sickle-shaped red blood cells
Hemolytic anemia due to premature destruction of sickled cells
Vaso-occlusion due to sickled cells blocking small blood vessels
Chronic organ damage from repeated vaso-occlusion and ischemia
Splenic sequestration and potential functional asplenia
Increased risk of gallstone formation
A Silent Hero Vows to Conquer Sickle Gallstones
What are the clinical features of thalassaemia?
Severe anemia: Profound fatigue, weakness, pale skin
Failure to thrive and growth retardation in children
Enlarged liver and spleen (hepatosplenomegaly)
Jaundice
Bone deformities, especially in the face (frontal bossing, prominent cheekbones)
Increased susceptibility to infections
Iron overload complications (without proper management):
Heart problems (heart failure, arrhythmias)
Liver cirrhosis
Endocrine disorders (diabetes, thyroid problems, growth hormone deficiency)
Osteoporosis
Delayed puberty
Some Friends Eat Jelly Beans In Interesting, Oval Dishes
What are the pathological features of thalassaemia?
Defective production of beta-globin chains
Ineffective erythropoiesis (red blood cell production)
Extramedullary hematopoiesis leading to hepatosplenomegaly
Hemolytic anemia
Iron overload in various organs due to repeated blood transfusions
Expansion of bone marrow cavities, leading to skeletal deformities
Increased gastrointestinal iron absorption
Don't Ignore Everyone's Heavy Iron Emphasis
What is the difference between sickle cell and thalassaemia
Sickle cell anemia is characterized by abnormal hemoglobin that causes red blood cells to sickle, while thalassemia major involves insufficient production of normal hemoglobin components
Define anaemia
A condition characterized by a deficiency of red blood cells or hemoglobin in the blood, leading to reduced oxygen transport to the body's tissues.
What mutation occurs in sickle cell disease
Sickle cell disease is caused by a single point mutation in the HBB gene, which codes for the β-globin subunit of hemoglobin.
The mutation changes the 6th codon of the β-globin gene from GAG to GTG.
This results in the substitution of glutamic acid (a hydrophilic amino acid) with valine (a hydrophobic amino acid) at the 6th position of the β-globin chain.
What effects does the sickle cell mutation have on protein structure
The mutated hemoglobin is called hemoglobin S (HbS).
The substitution of a hydrophilic amino acid with a hydrophobic one creates a sticky patch on the surface of the hemoglobin molecule.
Under low oxygen conditions, this sticky patch allows hemoglobin molecules to aggregate and form long, rigid polymers.
What affect does the sickle cell mutation have on RBC shape
The polymerization of hemoglobin S distorts the shape of red blood cells.
Instead of the normal biconcave disc shape, affected red blood cells become crescent or sickle-shaped.
What is the functional consequences of sickle cell mutation
Sickle-shaped cells are less flexible and can obstruct small blood vessels, leading to vaso-occlusion.
These cells are also more fragile, resulting in premature destruction (haemolysis).
The abnormal hemoglobin has a reduced ability to carry oxygen effectively.
What are the effects of oxygen deprivation
Tissue ischemia: When sickled red blood cells occlude blood vessels, the tissues supplied by those vessels become deprived of oxygen (ischemia).
Cell death: Prolonged oxygen deprivation leads to cell death in the affected tissues.
Inflammation: The ischemia-reperfusion injury that occurs when blood flow is restored triggers an inflammatory response.
Chronic organ damage: Repeated episodes of vaso-occlusion and ischemia lead to progressive organ dysfunction and damage.
What are the problems occur to the brain in sickle cell anaemia caused by vasocclusion.
Stroke
Silent cerebral infarcts
Cognitive impairment
What are the problems occur to the lungs in sickle cell anaemia caused by vasocclusion.
Acute chest syndrome
Pulmonary hypertension
Chronic lung disease
What are the problems occur to the heart in sickle cell anaemia caused by vasocclusion.
Cardiomegaly
Myocardial infarction
Heart failure
What problems can occur in the kidneys in sickle cell anaemia caused by vasocclusion.
Renal infarction
Chronic kidney disease
Proteinuria
What problems can occur in the liver in sickle cell anaemia caused by vasocclusion.
Hepatic infarction
Hepatomegaly
Liver fibrosis
What problems can occur in the Spleen in sickle cell anaemia caused by vasocclusion.
Splenic infarction
Functional asplenia
Increased susceptibility to infections
What problems can occur in the Bone in sickle cell anaemia caused by vasocclusion.
Avascular necrosis (especially of the hip)
Osteomyelitis
Chronic bone pain
What problems can occur in the eyes in sickle cell anaemia caused by vasocclusion.
Retinopathy
Retinal detachment
Vision loss
What problems can occur in the skin in sickle cell anaemia caused by vasocclusion.
Leg ulcers
Poor wound healing
What problems can occur in the genitourinary system in sickle cell anaemia caused by vasocclusion.
Priapism
Renal papillary necrosis
what is Priapism
Priapism is a medical condition characterized by a prolonged and often painful erection that lasts for more than four hours and occurs without sexual stimulation.
List the functions of the spleen
Blood Filtration
Immune function
Blood cell storage and release
Iron recycling
Haematopoiesis
Blood volume regulation
Removal of abnormal cells
Production of Opsonins
FIBer Helps A Really Big Opossum
Describe the role of the spleen in blood filtration
Removes old, damaged, and abnormal red blood cells from circulation
Filters out pathogens and foreign particles from the blood
Describe the role of the spleen in immune function
Houses antibody-producing lymphocytes in the white pulp
Produces antibodies and components of the immune system
Contains macrophages that remove antibody-coated bacteria and blood cells
Facilitates interactions between antigen-presenting cells and lymphocytes
Describe the role of the spleen in blood cell storage and release
Stores up to 1/3 of the body's platelets
Stores a reserve of red blood cells
Can release stored blood cells into circulation when needed (e.g. during hemorrhage)
Describe transient skin flora
Colonizes the superficial layers of the skin
More easily removed by routine hand hygiene
Often acquired through contact with patients or contaminated surfaces
Does not usually multiply on the skin but can survive temporarily
More frequently associated with healthcare-associated infections
Examples include various bacteria picked up from the environment
Describe resident skin flora
Resides under the superficial cells of the stratum corneum and on the skin surface
More difficult to remove through hand hygiene
Includes species like Staphylococcus epidermidis, other coagulase-negative staphylococci, and corynebacteria
Has protective functions like microbial antagonism and competition for nutrients
Generally less likely to cause infections
How can normal skin flora become pathogenic
Damage to the skin barrier can allow normally harmless bacteria to enter deeper tissues and cause infection
Immunosuppression of the host can allow normally benign bacteria to proliferate and cause disease
Changes in skin conditions (e.g. increased moisture) can promote overgrowth of certain species
Some resident flora can cause infections if introduced to sterile body sites or non-intact skin (Entry into sterile sites)
Certain strains of normally commensal bacteria may have increased virulence factors
Changes in the balance of the skin microbiome can allow potential pathogens to dominate
In summary, while the normal skin flora is generally harmless or even beneficial, changes in the host or microbial community can lead to pathogenic behaviour from these typically commensal organisms.
DICE Can Change
Describe the morphology of Staphylococcus
Staphylococci appear as spherical (cocci) bacteria
They typically occur in grape-like clusters
They may also be seen in pairs or short chains
Describe the staining characteristics of Staphylococcus
Staphylococci are Gram-positive, meaning they retain crystal violet stain and appear purple/blue when Gram stained
They stain uniformly (no internal structures visible)
Describe some common features of staphylococcus under the microscope
Size:
Individual cocci are approximately 0.5-1.5 μm in diameter
Arrangement:
The characteristic grape-like clusters are due to cell division occurring in multiple planes
Clusters typically contain 5-30 individual cocci
Other features:
Non-motile (do not move)
Non-spore forming
List the common infections associated with sickle cell
Pneumococcal infections: Streptococcus pneumoniae is one of the most common and serious pathogens in sickle cell disease patients, especially children.
Salmonella infections: Salmonella species are frequently associated with osteomyelitis in sickle cell patients.
Staphylococcal infections: Staphylococcus aureus is another common cause of infections, particularly osteomyelitis.
Urinary tract infections: These were found in about 3.9% of sickle cell patients in one study.
Bloodstream infections (bacteremia): More common in sickle cell patients, especially children.
Respiratory infections: Including pneumonia caused by various pathogens.
Meningitis: Particularly pneumococcal meningitis in children.
Osteomyelitis: Bone infections, often caused by Salmonella or Staphylococcus.
Sepsis: Severe systemic infections are more common in sickle cell patients.
Parvovirus B19 infection: Can cause aplastic crisis in sickle cell patients.
Malaria: In regions where malaria is endemic, it can be a significant problem for sickle cell patients.
Please Save Some Umbrellas Before Rain Makes Our Shoes Pristine Muddy
Explain the mechanism of damage to the spleen seen in sickle cell disease
Vaso-occlusion: Sickled red blood cells can block small blood vessels in the spleen, leading to repeated episodes of ischemia and infarction.
Congestion: Sickled cells can get trapped in the spleen's filtration beds, causing congestion and enlargement (splenomegaly).
Sequestration: Acute splenic sequestration crises can occur when large amounts of blood become trapped in the spleen, causing rapid enlargement and a drop in circulating blood volume.
Progressive damage: Over time, repeated episodes of vaso-occlusion and infarction lead to scarring and gradual loss of splenic function (functional asplenia).
Autosplenectomy: In many SCD patients, especially those with HbSS genotype, the spleen gradually shrinks and becomes non-functional by early childhood due to repeated damage.
Why does spleen damage from sickle cell disease predisposes to infections with encapsulated bacteria:
Loss of filtration function: The spleen normally filters blood to remove pathogens, especially encapsulated bacteria. Loss of this function increases susceptibility to these infections.
Impaired immune response: The spleen plays a crucial role in producing IgM antibodies, which are important for opsonizing encapsulated bacteria. Decreased splenic function leads to reduced IgM levels.
Reduced memory B-cells: The spleen is important for maintaining a population of memory B-cells that respond quickly to encapsulated bacteria. Loss of splenic function reduces this population.
Impaired phagocytosis: Splenic macrophages are important for phagocytosing opsonized bacteria. Their loss reduces the body's ability to clear these pathogens.
Specific vulnerability: Encapsulated bacteria like Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis are particularly challenging for the immune system without splenic function, as the capsule provides protection against other immune mechanisms.
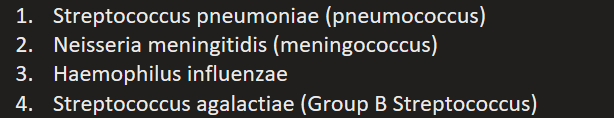
Name 4 types of encapsulated bacteria capable of causing severe infection.
Streptococcus pneumoniae (pneumococcus)
Neisseria meningitidis (meningococcus)
Haemophilus influenzae
Streptococcus agalactiae (Group B Streptococcus)