5.1-5.3 Redox reactions and Oxidation and reduction
Oxidation is the loss of electrons, gain of oxygen
Reduction is gain of electrons, loss of oxygen
^^u can remember this by OILRIG^^
^^Oxidation is Loss of electrons Reduction is Gain of electrons^^
sometimes oxidation and reduction happen together this reaction or process is called Redox Reactions.
Redox reactions can also be defined in terms of electron transfer
Oxidation is a reaction in which an element, ion or compound loses electrons
- The oxidation state of the element is increased
- This can be shown in a half equation, e.g. when silver reacts with chlorine, silver is oxidised to silver ions:
- Ag → Ag+ + e-
Reduction is a reaction in which an element, ion or compound gains electrons
- The oxidation state of the element is decreased
- This can be shown in a half equation, e.g. when oxygen reacts with magnesium, oxygen is reduced to oxide ions:
O2 + 4e- → 2O2-
- Oxidation and reduction in terms of electron transfer can be remembered by the mnemonic 'OIL RIG': Oxidation Is Loss of electrons, Reduction Is Gain of electrons
Mnemonic to remember oxidation and reduction in terms of electron transfer
\n \n
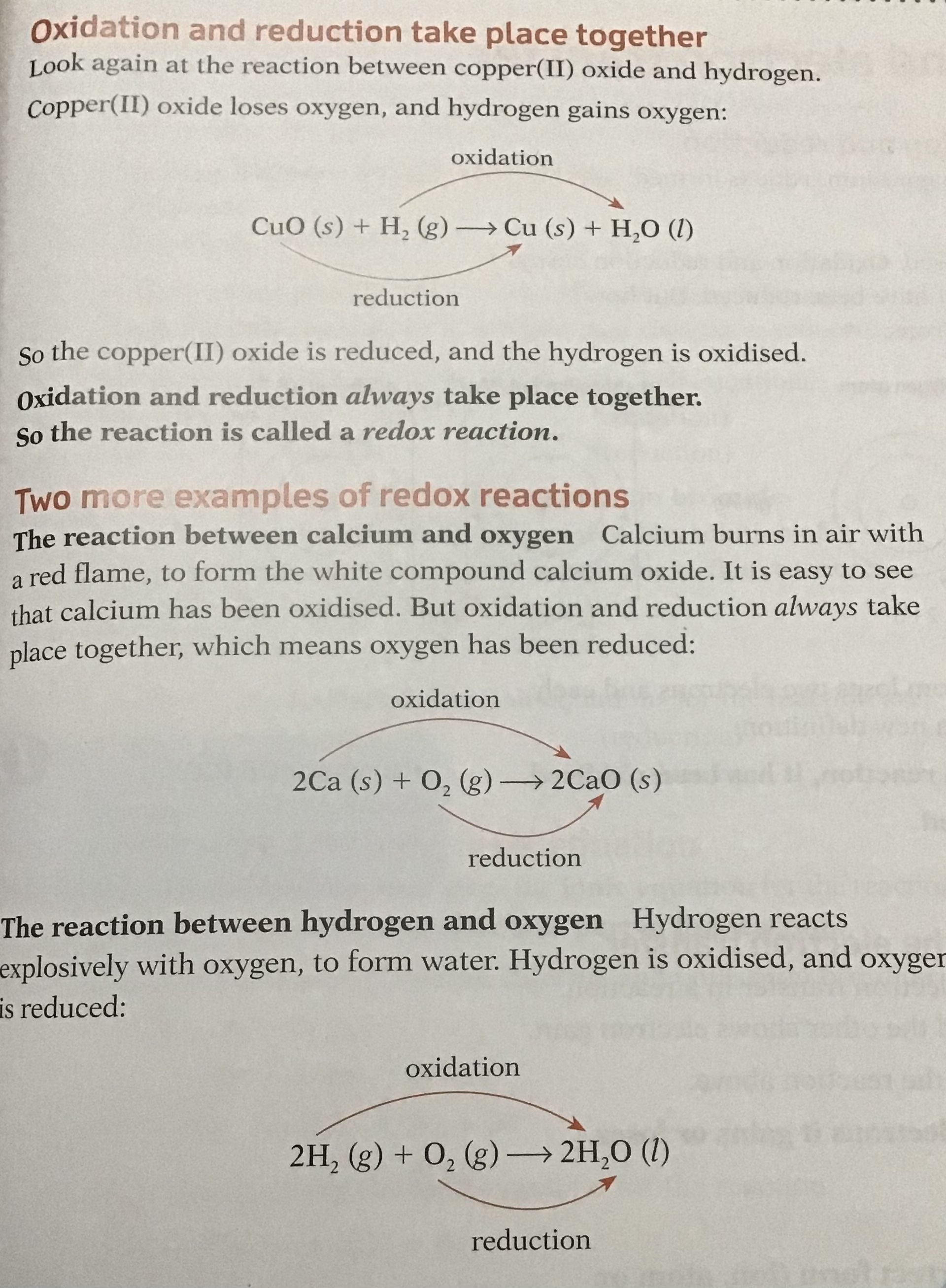
Names using oxidation states
Transition elements can bond in different ways by forming ions with different charges
When naming, the charge on the ion is shown by using a Roman numeral after the element's name
- e.g. iron can form ions with a 2+ charge, called iron(II) ions or a 3+ charge, called iron(III) ions
The Roman numeral is the oxidation state of the element
When iron reacts with oxygen to form iron oxide, the formula depends on the oxidation state of the iron ions
- The compound where iron has a 2+ charge has the formula FeO and is called iron(II) oxide
- The compound where iron has a 3+ charge has the formula Fe2O3 and is called iron(III) oxide
Oxidation number
The oxidation state (also called oxidation number) is a number assigned to an atom or ion in a compound which indicates the degree of oxidation (or reduction)
The oxidation state helps you to keep track of the movement of electrons in a redox process
It is written as a +/- sign followed by a number (not to be confused with charge which is written by a number followed by a +/- sign)
E.g. aluminium in a compound usually has the oxidation state +3
Assigning the oxidation state
Oxidation state refers to a single atom or ion only
The oxidation number of a compound is 0 and of an element (for example Br in Br2) is also 0
The oxidation number of oxygen in a compound is always -2 (except in peroxide R-O-O-R, where it is -1)
For example in FeO, oxygen is -2 then Fe must have an oxidation number of +2 as the overall oxidation number for the compound must be 0
- Table to show some common oxidation states of elements within compounds
- Example redox equation: electron loss/gain and oxidation state
- zinc + copper sulphate → zinc sulphate + copper
- Zn + CuSO4 → ZnSO4 + Cu
Writing all of the ions present and including state symbols we get:
- Zn(s) + Cu2+(aq) + SO42-(aq) →Zn2+(aq) + SO42-(aq) + Cu(s)
The spectator ions (those that do not change) are SO42-(aq), removing these we can write the ionic equation as:
- Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s)
By analysing the ionic equation, we can split the reaction into two half equations by adding in the electrons to show how the changes in charge have occurred.
It then becomes clear that zinc has been oxidised as its oxidation state has increased from 0 in Zn to +2 in Zn2+and it has lost electrons:
- Zn(s) → Zn2+(aq) + 2e-
Copper ions have been reduced as the oxidation state has decreased from +2 in Cu2+ to 0 in Cu and they have gained electrons:
- Cu2+(aq) +2e- → Cu(s)
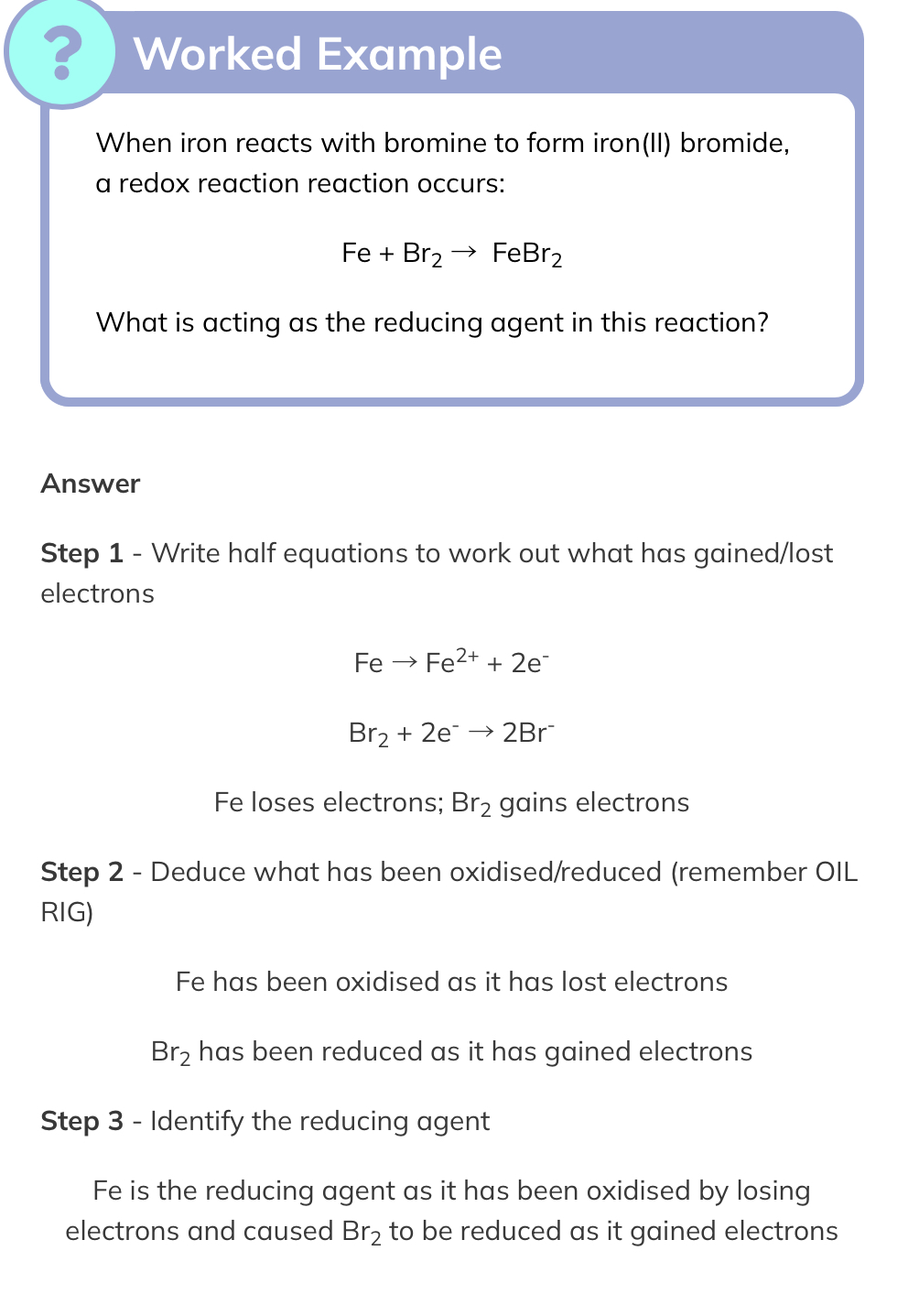
Oxidation and Reducing Agents
Oxidation Agent
- A substance that oxidises another substance, in so doing becoming itself reduced
- An oxidising agent gains electrons as another substance loses electrons
- Common examples include hydrogen peroxide, fluorine and chlorine
Reducing agent
- A substance that reduces another substance, in so doing becoming itself oxidised
- A reducing agent loses electrons as another substance gains electrons
- Common examples include carbon and hydrogen
- The process of reduction is very important in the chemical industry as a means of extracting metals from their ores
Example
CuO + H2 → Cu + H2O
In the above reaction, hydrogen is reducing the CuO and is itself oxidised as it has lost electrons, so the reducing agent is therefore hydrogen:
H2 → 2H+ + 2e-
The CuO is reduced to Cu by gaining electrons and has oxidised the hydrogen, so the oxidising agent is therefore copper oxide
Cu2+ +2e- → Cu