Unit 3 - Elements and the Periodic Table
Elements, Compounds, and Mixtures
- Changes
- Physical Changes - Don’t produce a new substance
- Chemical Changes - Produces a new substance
- Matter Classification
- Pure Substances
- Can’t be broken into simpler compounds without going through a chemical change
- Made of atoms that are chemically bonded to each other
- Elements - Pure substances made of only 1 type of atom
- Compounds - Pure substances made of 2+ types of atoms
- Have fixed ratios between components
- Mixtures
- Mixing 2+ substances that are NOT chemically combined
- Can be separated through physical means
- Distillation - Separating components in a mixture through the use of their differing boiling points
- Chromatography - Separating components using differences in their ability to pass through substrates
- Don’t have fixed ratios between components
- Types of Mixtures
- Homogeneous Mixture - The components combined are indistinguishable
- Heterogeneous Mixture - The components combined are distinguishable
Atomic Numbers and Electron Configurations
- Quantum Orbitals
- Orbitals - Location in an atom where an electron could be
- An atom can have any number of orbitals depending on the number of electrons they have
- Each orbital can hold 2 electrons
- Quantum Number - Describes the location of an electron / describes the orbital
- Three main quantum numbers used to describe orbitals: “N“, “L“, and “M“
- N - Principal quantum number; describes the size of the orbital
- Must be >0
- You can think of this as what ring in the Bohr model the orbital coincides with
- L - Angular momentum quantum number; describes the orbital shape
- Can be spherical, dumbbell/peanut, clover, etc shaped
- Can be between 0 → N-1
- M - Magnetic quantum number; describes orientation of the orbital
- Can be between -L → +L
- Shells & Subshells
- Electron shell - A group of orbitals with the same principle quantum number (N)
- Shells are filled consecutively from the center/lowest energy orbitals outward
- Different shells can hold different numbers of electrons
- Full shells are the most stable
- Electron subshells - A group of orbitals with the same principle quantum number (N) AND angular momentum quantum number (L)
- Subshell Classifications
- L = 0 → S Orbital
- L = 1 → P Orbital
- L = 2 → D Orbital
- L = 3 → F Orbital
- The number of different values the magnetic quantum number (M) can be is equal to the number of subshells of a certain classification
- The number of orbitals is equal to the number of different combinations of N, L, and M (Can be calculated with N^2)
- To calculate the number of electrons a shell can hold, you just double this number, since each orbital can hold 2 electrons
- This can also be calculated with the formula 2N^2
- Electron Configuration - How electrons are positioned in an atom
- Orbital Notation - A diagram that shows shells, subshells, and orbitals using lines & arrows
- Lines represent orbitals
- Numbers & letters at the bottom represent the name of the orbital
- Arrows represent electrons
- Upward and downward arrows represent a M subscript s value of either 1/2 or -1/2
- Pauli Exclusion Principle - No 2 electrons can have identical quantum numbers
- A fourth quantum number, M subscript s represents the quantum spin of a number
- Can have a value of either -1/2 and 1/2
- Only 2 values → only 2 electrons can be in a orbital, otherwise at least 1 pair of electrons will have identical quantum numbers
- Hund’s Rule - Electrons are placed in individual orbitals before being paired
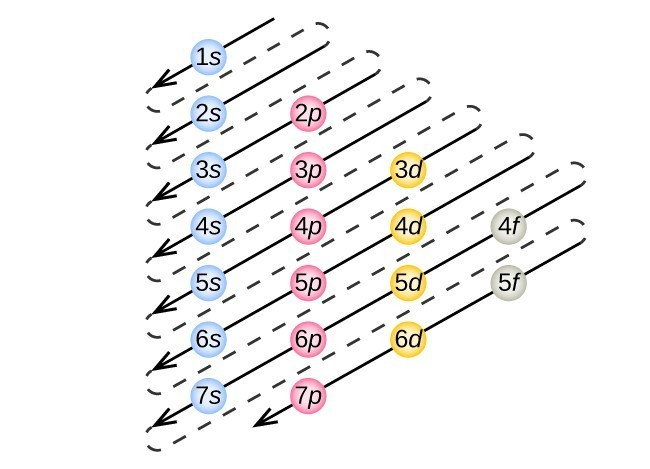
- Aufbau Principle - Electrons fill orbitals from lowest energy → highest energy
- This means electrons fill from lower N to higher N
- D and F are the exception; 3d has higher energy than 4s, so 4s will fill before 3d.
- Follow the diagonal rule to determine order in which orbitals are filled
The History and Arrangement of the Periodic Table
- Antoine Lavosier
- Wrote “Elementary Treatise of Chemistry“ in 1789
- Considered the world’s first modern chemistry textbook
- Classified elements into 4 groups:
- Acid-making
- Gas-like
- Wrongly classified light & heat as elements
- Metallic
- Earthy
- Almost entirely made up of compounds
- John Dobereiner
- Arranged elements w/ similar properties into triads (groups of 3)
- Difference between mass of elements 1 & 2 is about equal to difference in mass between 2 & 3
- John Newlands
- Arranged elements by atomic mass
- Established “law of octaves“
- Repeating pattern of similar properties every 8 elements
- Dimitri Mendeleev
- Created the first iteration of the modern periodic table
- Arranged elements by atomic mass
- Organized table rows/columns by chemical properties
- Henry Moseley
- Arranged elements by atomic number
- Account for variation in natural isotopes
- Periodic Table
- Organized by atomic number (number of protons)
- Columns have similar chemical properties due to having the same number of valence (outer) electrons
- Each row is a new shell
- Periods - A row on the periodic table
- Atomic number increases from left to right
- Chemical properties systematically change
- Groups/Families - A column on the periodic table
- All elements in groups have similar chemical properties
- Cells - Give information about an element
- Atomic number
- Atomic mass
- Atomic symbol
- Element name
- Elements
- Natural: Elements 1-94
- Man-Made: Elements 95-118
- Metals: Left of the “staircase“ except hydrogen
- Malleable
- Ductile
- Conduct heat & electricity
- Mostly solids
- Semi-Metals/Metalloids: The “staircase”
- Properties of both groups
- Non-Metals: Right of the “staircase“ plus hydrogen
- Brittle
- Poor Conductors
- Can be any state
- Main Group Elements
- Alkali Metals - Group 1
- Silver colored
- Soft
- Highly reactive with water/oxygen
- Oxidizes in air
- Alkaline Earth Metals - Group 2
- Silver colored
- More brittle than alkaline metals
- Somewhat reactive
- Low density, melting, and boiling points
- Halogens - Group 17
- Highly reactive w/ metals
- Form salts
- Toxic to most organisms
- Mostly occur as diatomic molecules
- Noble Gases - Group 18
- Stable; don’t bond w/ other atoms
- Non-flammable
- Extremely low boiling points
- Used in lights, produces colors when excited
- Transition Metals
- Form colored compounds
- Some have unique properties
- Some are magnetic
- Some are very reactive
- Inner Transition Metals
- Can be radioactive
- Lanthanides
- Actinides
Electrons and the Periodic Table
- Noble Gas Notation
- Using noble gases to represent filled shells in longhand electron configuration
- Separates valence and non-valence(core) electrons in an atom
- Valence Electrons
- The number of electrons on the outer shell of an atom
- Determines the chemical properties of the atom
- Correlated with the groups that the element is in in the periodic table
- Group number = number of valence electrons
- Determining Valence Electrons
- Periods 1-3
- Group number / highest S and P orbitals
- Periods 4+
- Highest S and P orbitals + partially filled d and f orbitals
- Periodic Table & Orbitals
- S-Block Elements - Elements in groups 1 & 2 + Helium
- Has valence electrons in the S orbital
- P-Block Elements - Elements in groups 13 → 18 - Helium
- Rows 1-3
- Has valence electrons in the S and P orbitals, with the last added electron being in the P orbital
- Rows 4+
- Has valence electrons in the S, D, and P orbitals, with “N“ of the D subshell being 1 less than the N of the S & P subshells and the last added electron being in the P orbital
- D-Block Elements - Elements in groups 3 → 12 + Lutetium and Lawrencium
- Has valence electrons in the S and D orbitals, with “N“ of the D subshell being 1 less than the N of the S subshell and the last added electron being in the D orbital
- F-Block Elements - Lanthanides & Actinides - Lutetium and Lawrencium
- Has valence electrons in the S and F orbitals, with “N“ of the F subshell being 2 less than the N of the S subshell and the last added electron being in the F orbital
- Exceptions
- Chromium
- Predicted - [Ar] 4s^2 3d^4
- Actual - [Ar] 4s^1 3d^5
- Often D & F block elements that are transition metals
- Happens because electrons fill lowest energy shell
Periodic Trends
- Atomic Radius - 1/2 the distance between two identical atoms in a diatomic molecule
- Increases down a group
- Decreases across a row
- More protons → electrons are pulled slightly closer together
- Ionic Radius - Measure of the size of an ion
- Anion - Negative ions (atoms that gain electrons)
- Larger; More electrons cause more electron repulsion
- Cation - Positive ions (atoms that lose electrons)
- Smaller; less electrons cause less electron repulsion
- Increase down a group
- Decrease for cations across a row
- Decrease for anions across a row
- Increase when switching from cations to anions across a period
- Ionization Energy - The energy required to remove an electron from an atom in a gas phase
- Changes based
- Nuclear charge
- Distance from nucleus
- The number of already removed electrons
- First Ionization Energy - Energy needed to remove 1 electron from an atom
- Second ionization energy - amount of energy to remove another electron after the first one is removed, etc
- Main Group Elements
- Increases across periods
- Decreases down groups
- Decreases between groups 2 & 13 and groups 15 & 16
- Electron Affinity - Energy required to add an electron to a neutral atom in a gas phase
- Decreases across a period
- Increases down a group
- Electronegativity - How much an atom attracts other electrons from other atoms
- Increase across a period
- Decrease down a group