skeletal muscles
1/62
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
63 Terms
skeletal muscle
- under the control of the voluntary nervous system
- makes up the bulk of the body muscle in vertebrates
- is attached to the skeleton
- controlled by motor neurones
- motor neurones interact with muscles at a neuromuscular junction
gross structure of skeletal muscle
- skeletal muscle is attached to bones by tendons and is made up of bundles of muscle fibres a few cm long
- muscle fibres are fused muscle cells (with several nuclei and lots of mitochondria)
- surrounded by a cell membrane called the Sarcolemma which is strengthened by a thin outer layer of polysaccharide and collagen fibres
- within each muscle fibre are bundles of protein filaments called myofibrils
- since muscle fibres are filled with myofibrils, they have their cytoplasm (called sarcoplasm) around the outside of the cell
- they also contain myoglobin and lots of mitochondria
how do muscles move the skeleton?
- muscles act in antagonistic pairs against an incompressible skeleton
- the two antagonistic muscles that bend the arm around the elbow joint are called the biceps and triceps
what is a muscle fascicle?
a bundle of skeletal muscle fibres
muscle fibres
- muscle is made of individual cells fused together which form muscle fibres
- the fibres are multinucleated (share many nuclei)
- sarcolemma surrounds the muscle fibre
- muscle fibre contains sarcoplasm
- network of membranes - sarcoplasmic reticulum
- many mitochondria
- within the muscle fibre are many myofibrils, which give the cell its striated appearance
sarcolemma
plasma membrane
myofibrils
the sarcoplasmic reticulum surrounds the myofibrils and stores and releases Ca2+
sarcoplasmic reticulum
- the network of tubes surrounding the myofibrils
- its role is to absorb Ca2+ during relaxation and release Ca2+ during contraction
t-tubules
- the sarcolemma has pores with membrane bound tubes called t-tubules
- lead down to the myofibrils
- run against the sarcoplasmic reticulum that surrounds the myofibrils
- allow the conduction of nerve impulses
- carry APs deep into the muscle fibre causing the sarcoplasmic reticulum to release Ca2+
sarcomere
the distance between two Z lines
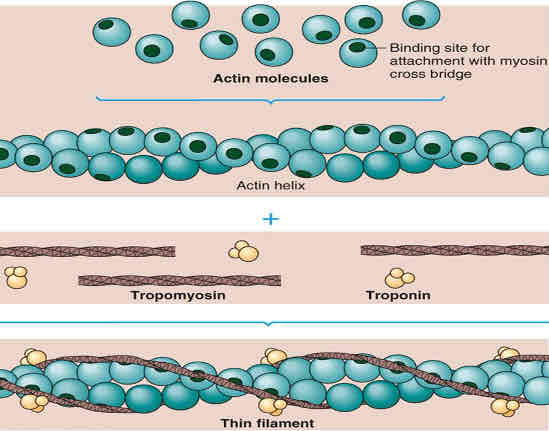
actin
thin filaments
2 actin strands twisted around each other
each actin subunit has a binding site for myosin heads
troponin is attached to tropomyosin and has a binding site for calcium ions
tropomyosin blocks the myosin head binding site at rest
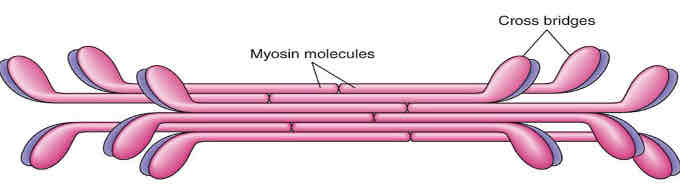
myosin
- thick filament, made from many myosin proteins wound together
- a fibrous myosin protein forms the tail
- myosin globular heads contain: ATP hydrolase enzyme, actin binding site, and ATP binding site
Z line
a line that separates one sarcomere from another
I bands
appear lighter as only actin filaments are present
A bands
appear dark as there is overlapping of both actin and myosin
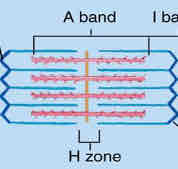
H zone
only myosin filaments are present
M line
central line of the sarcomere where myosin filaments are anchored
sliding filament mechanism
actin and myosin can bind together and slide over each other
the interlocking structure of the myosin and actin filaments allows them to slide past one another
this reduces the length of the sarcomere, causing the muscle to contract
at the same time the banding pattern of the sarcomere changes; light bands, formed by actin, shrink as the filaments become more interlocked
during an impulse ATP hydrolase hydrolyses ATP to form ADP + Pi → this pulls the myosin head back, creating potential energy
neuromuscular junction
- synapse between motor neurone and muscle fibres
- impulse
- synaptic vesicles fuse with membrane
- acetylcholine diffuses
- binds to receptor in the postsynaptic membrane (sarcolemma) of the muscle fibre
- sodium ions enter, depolarising the sarcolemma (membrane)
- AP travels along sarcolemma and down the T-tubules that surround the myofibrils
- acetylcholine is broken down by acetylcholinesterase (so the muscle is not over-stimulated)
- choline and acetyl return back to neurone where they recombine using ATP from mitochondria
- the muscle fibre is the end of the AP
similarities between neuromuscular junction and cholinergic synapse
neurotransmitters that are transported via diffusion
have receptors, that cause an influx of sodium ions (when a NT is bound)
use of sodium-potassium pump to repolarise the axon membrane
use enzymes to break down the neurotransmitter
differences between neuromuscular junction and cholinergic synapse
- neuromuscular only excitatory whereas cholinergic may be excitatory or inhibitory
- neuromuscular only links neurones to muscles whereas cholinergic links neurones to neurones, or neurones to other effector organs
- neuromuscular only motor neurones are involved whereas cholinergic motor, sensory and relay neurones may be involved
- neuromuscular the AP ends here (it’s the end of a neural pathway) whereas cholinergic a new AP may be produced along another neurone (the postsynaptic neurone)
- neuromuscular acetylcholine binds to receptors on membrane of muscle fibres whereas cholinergic acetylcholine binds to receptors on membrane of postsynaptic neurone
how does an action potential trigger muscle contraction?
muscles are under nervous control
contraction is initiated at the neuromuscular junction: a junction between a motor neurone and muscle fibre
action potential
- AP across neuromuscular junction creates an influx of sodium ions
- sarcolemma depolarises, the AP travels down the T-tubules
- this causes calcium ion channels in the sarcoplasmic reticulum to open
- calcium ions diffuse out of SR and bind to troponin
- this causes troponin to move the tropomyosin away from the myosin binding sites on actin
- myosin can now bind to actin and form a “cross bridge”
the cycle of actinomyosin bridge formation
- at rest the binding site on the actin is covered by the protein tropomyosin → tropomyosin is attached to troponin
- when calcium is released from the endoplasmic reticulum it binds to the troponin
- this causes it to change shape, shifting the tropomyosin and revealing the binding site
- the myosin head attaches to the binding site on the actin, this forms a cross-bridge
- the myosin head then bends backwards on itself, pulling the actin → the ADP is released from the myosin head → this is called a power stroke
- the ATP is hydrolysed by ATP synthase (this is activated by the calcium) and the energy is stored in the myosin head for use later
- the myosin head returns to its original position
- as long as the calcium is still bound to the troponin, and the tropomyosin is still out of its original position, the active sites are exposed
- the myosin head can bind on to another binding site and repeat the process again
- in this way there will be lots of small power strokes moving the actin filament along a little bit each time
role of ATP in myofibril contraction
- allows binding of myosin to actin to form a cross bridge
- provides energy to move the myosin head to create a power stroke
- detachment of myosin from actin
phosphocreatine
- found in muscles
- used to regenerate ATP in anaerobic conditions
- provides energy and Pi to make ATP from ADP in substrate level phosphorylation
- the reaction is catalysed by the enzyme creatine kinase
- stored ATP and phosphocreatine provide energy for maximum muscle power for 10-15 seconds
- found in fast twitch muscle fibres
types of muscle fibres
fast twitch and slow twitch
slow skeletal muscle fibres
endurance: contract slowly, contract for longer ‘sustained muscle contraction’
high resistance to fatigue
muscle contractions are not as powerful
adapted for aerobic respiration
many mitochondria present for the production of lots of ATP
small amounts of glycogen present
a rich blood supply: deliver O2 and glucose
lots of myoglobin
how are slow skeletal muscle fibres adapted to their function?
adapted for aerobic respiration
large myoglobin store
rich supply of blood vessels to deliver oxygen and glucose
fast skeletal muscle fibres
- contract quickly: immediate, rapid muscle contractions, but for short lengths of time
- low resistance to fatigue, fatigue easily
- powerful muscle contractions
- depend mainly on glycolysis for the (anaerobic) production of ATP
- more enzymes regulating glycolysis (because more uses anaerobic respiration)
- few mitochondria present
- large amounts of glycogen present
- thicker and more myosin present
- lots of phosphocreatine present
- e.g. sprinting, weight lifting
how are fast skeletal muscle fibres adapted to their function?
thicker and more myosin filaments
have larger glycogen supply to provide a source of metabolic energy, which is hydrolysed for aerobic and anaerobic respiration
higher concentration of enzymes involved in anaerobic respiration (glycolysis) to provide ATP rapidly
high levels of phosphocreatine which can generate ATP from ADP in anaerobic conditions to provide energy for muscle contraction
myoglobin
protein which stores oxygen
what are the three types of muscle found in the body?
skeletal, smooth and cardiac
are skeletal muscles under voluntary or involuntary control?
voluntary control
are smooth muscles under voluntary or involuntary control?
involuntary control
are cardiac muscles under voluntary or involuntary control
involuntary control
what is the name of the cytoplasm in a muscle fibre?
sarcoplasm
what are the two main types of protein filament found in a myofibril?
actin and myosin
when the muscle contracts, what happens to the width of the A band?
stays the same
when the muscle contracts, what happens to the width of the I band?
becomes narrower
what is the role of phosphocreatine in providing energy for muscle contraction?
produces ATP in anaerobic conditions by substrate level phosphorylation
cardiac muscle
in heart (involuntary)
smooth muscle
in walls of most ‘tubular’ organs, e.g. intestines, blood vessels, reproductive system
main function is peristalsis’s
involuntary
myofibril structure
each myofibril is made of 2 types of protein filament: myosin and actin
tropomyosin
blocks the binding sites for myosin on the actin
troponin
is complexed with tropomyosin → troponin-tropomyosin complex
ultrastructure
the structure of the myofibril
why do skeletal muscle have glycogen granules?
as a store of glucose (glycogen can be hydrolysed to glucose) to be used in respiration to provide ATP
how does a fall in the pH of skeletal muscle tissue lead to a reduction in the ability of calcium ions to stimulate muscle contraction?
low pH changes shape of calcium ion receptors
fewer calcium ions bind to tropomyosin
fewer tropomyosin molecules move away
fewer binding sites on actin revealed
fewer cross-bridges can form
describe the roles of calcium ions and ATP in the contraction of a myofibril
1. calcium ions diffuse into myofibrils from sarcoplasmic reticulum
2. calcium ions cause movement of tropomyosin on actin
3. this movement causes exposure of the binding sites on the actin
4. myosin heads attach to binding sites on actin
5. hydrolysis of ATP on myosin heads causes myosin heads to bend
6. bending pulling actin molecules
7. attachment of a new ATP molecule to each myosin head causes myosin heads to detach from actin sites
what is the role of ATP in myofibril contraction?
reaction with ATP allows binding of myosin to actin
provides energy to move myosin head
explain why both slow and fast muscle fibres contain ATPase
hydrolysis of ATP
muscle contraction requires energy
use of ATP by myosin
what is the role of phosphocreatine in providing energy during muscle contraction?
phosphocreatine provides phosphate to make ATP
describe the role of tropomyosin in myofibril contraction
moves out of the way when calcium ions bind
allowing myosin to bind to actin to form a cross bridge
describe the role of myosin in myofibril contraction
head of myosin binds to actin and slides actin past
myosin detaches from actin and moves further along actin
this uses ATP
what happens to the length of the I band when the sarcomere contracts?
decreases
what happens to the length of the A band when the sarcomere contracts?
nothing
muscle contraction - stimulation of muscle
the nerve impulse arrives at the neuromuscular junction
acetylcholine is released into the synaptic cleft
an AP is produced in the sarcolemma of the muscle fibre
the AP is transmitted down t-tubules
causing the sarcoplasmic reticulum to release calcium ions
muscle contraction
6. calcium ions bind to troponin-tropomyosin complex which moves tropomyosin away from the actin
7. exposing its myosin binding sites
8. myosin heads bind to the exposed sites on the actin filaments (Pi is released from myosin head)
9. myosin heads changes position (powerstroke) and slides actin over the myosin (ADP is released from myosin head)
10. ATP bind to myosin head and breaks crossbridge
11. ATP hydrolysis provides the energy to reposition the myosin head, so it can bind again
12. process repeats
what is the importance of calcium ions in muscle contraction?
- they activate myosin ATP hydrolase which hydrolyses ATP to ADP + Pi
- binds troponin, causing tropomyosin to detach from actin filament uncovering myosin binding sites on actin which allows actin-myosin cross bridges to form
explain how ATP helps the myosin and actin filaments to slide over each other during the shortening of a muscle cell
energy source
to enable formation of actomyosin cross bridges
when happens to the appearance of the H zone when the the muscle contracts?
shorter
describe how stimulation of a muscle by a nerve impulse starts muscle contraction
calcium ions released by sarcoplasmic reticulum
bind to tropomyosin and displaces the tropomyosin
to reveal binding site on actin
myosin binds to exposed sites on actin and forms cross bridges between actin and myosin
activates ATP hydrolase
after death, cross bridges between actin and myosin remain firmly bound resulting in rigor mortis. explain what causes the cross bridges to remain firmly bound.
- respiration stops
- no ATP produced
- ATP required for separation of actin and myosin cross bridges
- so myosin heads remain bound to actin