Covalent bonding 2
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
What is electronegativity
The ability to attract electrons towards itself in a covalent bond
What happens to electronegativity across a peroid
increases
What happens to electronegativity down a group
decreases
What do chemists to measue electronegativity
Pauling scale
The higher the number of the Pauling scale what does that mean
The higher the number, the more electronegative the atom.
Hydrogen has similar electronegativity to what element and why
C because Hydrogen has no shielding
Most electronegative element is
F
What makes a bond polar
if difference in Pauling’s scale value > 0.5
What makes a bond more polar
The larger the difference in electronegativity
what bonding is the Compounds with a difference in electronegativity larger than 2.5 in compound
ioncally
what bonding is the Compounds with a difference in electronegativity smaller than 2.5 in compound
covalently
Elements with electronegativities larger than or equal to 2.12.12.1 tend to bond in elements
covalently
Elements with electronegativities smaller than or equal to 1.51.51.5 tend to bond
metallically
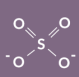
Sulfate structure
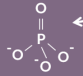
phosphate structure
cn^-
