The structure of polymer molecules and the random walk model
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
29 Terms
Equation for random walk
R0 ² = Na²
R0 ² = mean square end to end distance.
N = no. steps taken in random walk/monomer size
a² = number of monomers per molecule
Conditions under which Random walk applies:
Negligible monomer volume.
No interactions between monomers.
Successive steps uncorrelated.
Solvent in which conditions apply
Theta solvent
Good solvent
monomers repel one another/large volume
polymer structure more swollen than random walk with longer end-to-end distance
Poor solvent
Monomers attract each other hence stick together
polymer structure less swollen than random walk
Theta solvent (θ)
Monomers slightly attract each other
significant volume
hence 2 effects cancel each other out
Other examples of random walk
Diffusion
Statistical quantities
Financial markets
Flory Model
This is a model for a good solvent. We can guess this because the proportionality is R ~ N3/5 and the exponent 3/5 is greater than the random walk value of ½, so the chain is more swollen. (Remember the random model walk is for a theta solvent [or in a melt].)
![<p><span>This is a model for a </span><strong><span>good solvent</span></strong><span>. We can guess this because the proportionality is R ~ N<sup>3/5 </sup>and the exponent 3/5 is greater than the random walk value of ½, so the chain is more swollen. (Remember the random model walk is for a theta solvent [or in a melt].)</span></p>](https://knowt-user-attachments.s3.amazonaws.com/f1ec7db5-5fff-456d-87e2-59d09fceca9c.png)
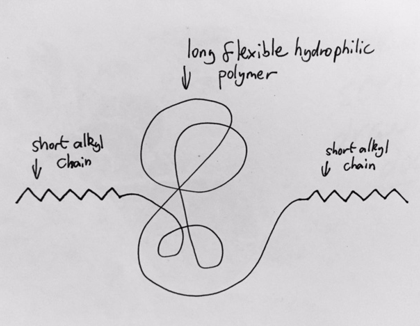
Is this polymer, if dissolved in water, likely to form a gel?
Yes
Short alkyl chain property:
Non-polar molecule so doesn’t dissolve in water so will want to separate. By moving hydrophobic molecule closer to H20 reduces hydrophobic interactions.
Alkyl chains form assemblies/i .e. cluster together -via cross-linking
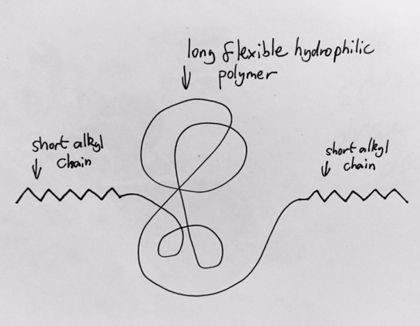
Essential requirement for formation of gel
cross-linking between molecules.
Helmholtz free energy of a system
max work that can be extracted from that system
Effect of decrease in entropy on extension and no . possible configurations
extension increases, and no. possible configurations decreases hence positive elastic restoring force i.e. material is elastic.
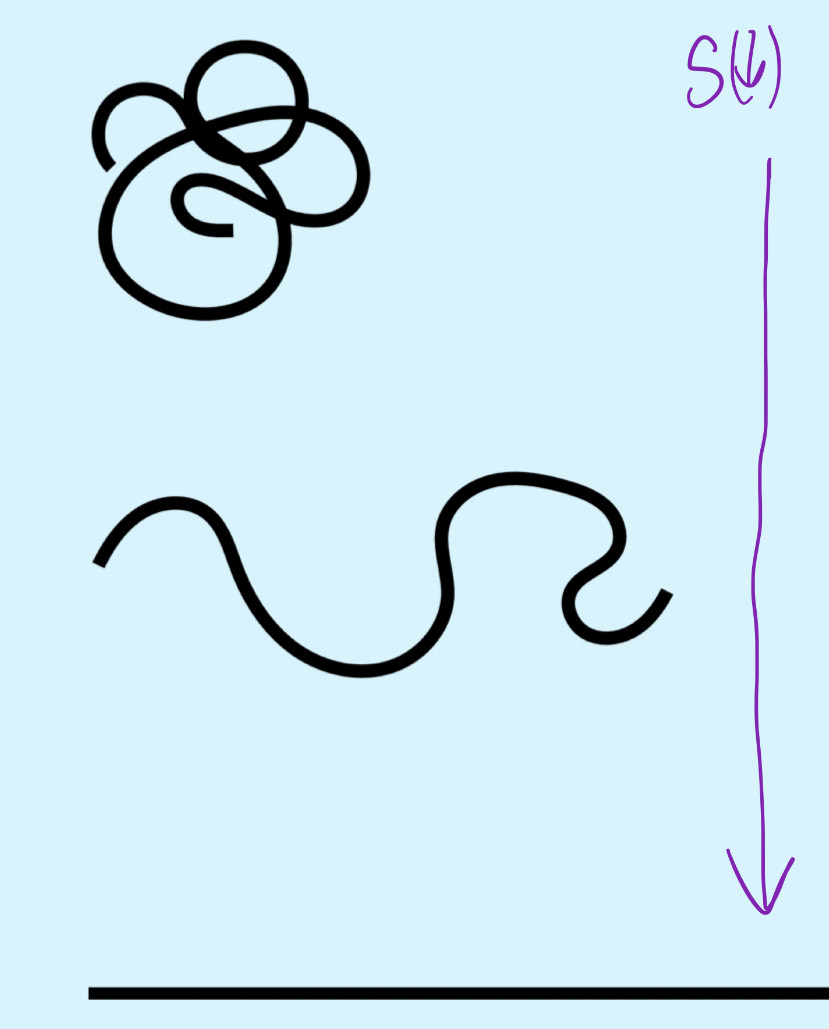
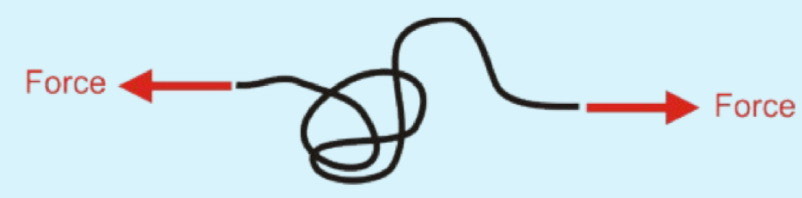
S-driven elasticity (Entropy driven elasticity)
Ideal polymer
Chains extend
Chemical bonds don’t stretch
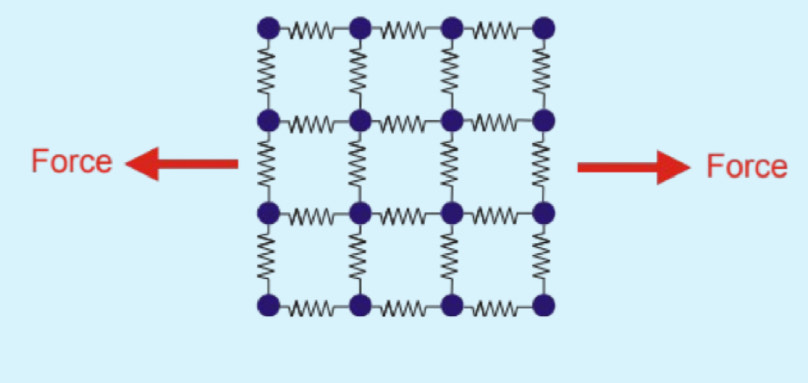
U-driven elasticity (Internal energy driven elasticity)
e.g. metal
Chemical bonds do stretch
2 different origins for elasticity:
In a crystalline material, capacity to do work stored by a higher potential energy within it.
Stretched rubber band no storage of potential energy- capacity to do work stored by lower configurational entropy within material.
How does elasticity arise in a crystalline material?
From change in internal energy(U) - caused by stretched bonds
How does elasticity arise in stretched rubber band?
chemical bonds do not stretch
BUT polymer chains within material unwind, when extended
so lower entropy state
Elastic restoring force in ideal polymer chain (random walk model)
thermal kinetic energy depends only on the temperature → doesn’t change
internal energy U → doesn't change on extension
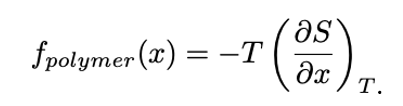
Elastic restoring force in Energy-elastic material (i.e. idealized metal, ceramic)
at constant temperature, the entropy is independent of extension, x.
BUT bonds between the atoms stretch, storing potential energy ⇒ changing U
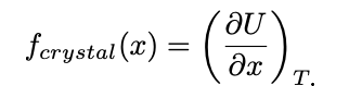
General equation for elastic restoring force:
A = Helmholtz free energy
U = Internal energy
S = Entropy
T = Temperature
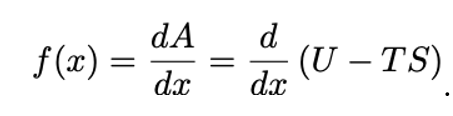
For a piece of metal in contact ,with its surroundings- reversible thermo = isothermal (temp doesn’t change)
Which can be broken up into its internal energy + entropy components.
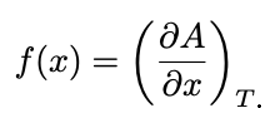
If a material is not thermally isolated
adiabatic so reversible not isothermal
What is the difference between a rubber and a gel?
Rubber = formed from cross-linking polymer melt
Gel = formed via cross-linking polymer solution
2 main macroscopic mechanical properties that characterize an elastomeric material?
Hyperelasticity
Very low stiffness (Young’s modulus)
What does configurational entropy generate?
A restoring force that contracts polymer size.
What occurs at thermal equilibrium ?
partial derivative of A (Helmholtz energy) with respect to R (T stays constant) = 0 i.e. A = maximized
Does the configurational entropy of polymer chain within sample of elastomeric polymeric materials increase/ decrease when stretched?
Entropy decreases
The uncoiling of the polymer chain upon stretching reduces no. potential config available so S decreases
Type of model for single polymer chain config
Random-walk-on-a-lattice
Type of model for gelation
Lattice percolation