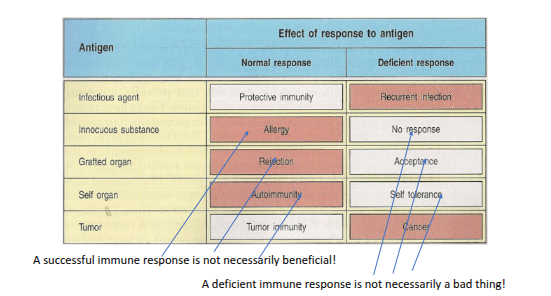
The ‘antigen’ driven immune response
1/69
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
70 Terms
Antigen driven immunity
What are the three signals required for T-cell activation?
Engagement of antigen with the T-cell receptor, co-stimulation, and receipt of cytokine stimulation
What is the first signal in T-cell activation?
Engagement of the antigen with the T-cell receptor (TCR)
What is the second signal in T-cell activation?
Co-stimulation, typically provided by interactions between co-stimulatory molecules on the T-cell and antigen-presenting cell.
What is the third signal in T-cell activation?
Cytokine stimulation, which directs T-cell proliferation and differentiation
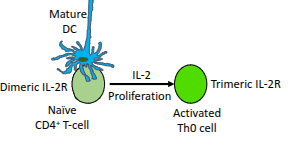
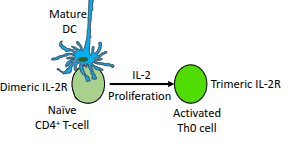
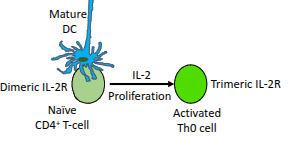
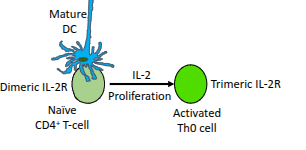
Which cytokine drives the proliferation of activated CD4⁺ T-cells?
Interleukin-2 (IL-2).
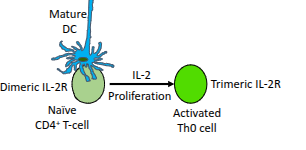
What happens to naïve CD4⁺ T-cells in the presence of IL-2?
They upregulate their IL-2 receptor (IL-2R), enhancing their responsiveness to IL-2.
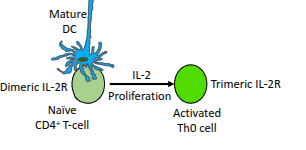
What type of cells are produced when a naïve CD4⁺ T-cell becomes an activated Th0 cell?
Resting effector cells.
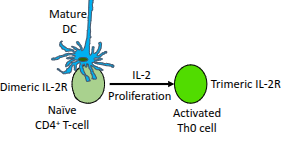
Name some effector T-helper subsets that can develop from Th0 cells.
Th1, Th2, Th17, Th9, Th22, and follicular helper T-cells (Tfh)
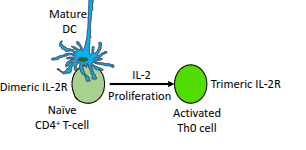
What role do mature dendritic cells (DCs) play in T-cell activation?
They present antigen and provide co-stimulation to activate naïve CD4⁺ T-cells
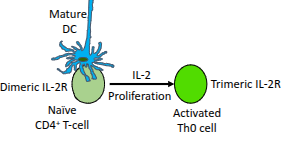
What is the functional difference between the dimeric and trimeric IL-2 receptor?
The trimeric IL-2 receptor has higher affinity for IL-2 than the dimeric form, enabling stronger proliferative signalling
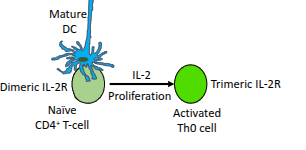
What determines which Th subset a CD4⁺ T-cell will differentiate into?
The polarising cytokines it encounters, the master gene regulator induced, and the signature effector cytokines it later produces
What characterises each major Th subset?
A distinct set of polarising cytokines, a specific master gene regulator, and a characteristic profile of effector cytokines.
What are polarising cytokines?
Cytokines that drive a naïve CD4⁺ T-cell towards a particular Th lineage.
What induces the expression of a master gene regulator during Th differentiation?
The specific polarising cytokines present during T-cell activation.
What is the role of the master gene regulator in Th differentiation?
It controls gene expression patterns that define the Th subset.
What are signature effector cytokines?
The characteristic cytokines produced by a fully differentiated Th subset.
What determines the polarising cytokines produced during T-cell activation?
The PAMP-induced activation of TLRs/NOD receptors and the nature of the dendritic cell and its associated peptide
How do PAMPs influence Th subset development?
They activate specific TLRs/NOD receptors on dendritic cells, shaping the cytokines produced.
How does the nature of the dendritic cell influence Th subset choice?
Different dendritic cell types and the peptides they present release different polarising cytokines, directing Th differentiation
Th1 effectors
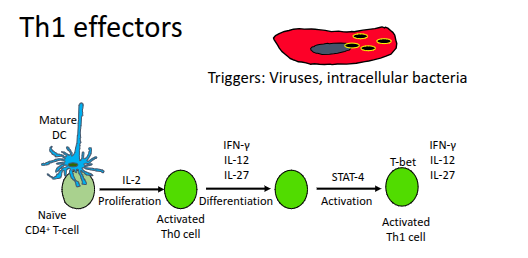
Which key cytokines are produced by Th1 cells?
IL-2, IFN-γ, and lymphotoxin (LT).
What is the role of IL-2 produced by Th1 cells?
It drives the proliferation of T-cells and B-cells
How do Th1 cells support CD8⁺ T-cells?
By providing T-cell help that enhances cytotoxic responses against intracellular pathogens.
How do Th1 cells support B-cells?
They help B-cells mount antibody responses against intracellular pathogens.
How do Th1 cells enhance macrophage function?
By increasing the production of reactive oxygen intermediates (ROIs) in macrophages
How does IFN-γ influence NK cells and macrophages?
It increases the expression of high-affinity Fcγ receptors on both cell types.
How does IFN-γ affect antibody class switching?
It promotes switching to IgG1 and IgG3, which are effective against intracellular pathogens in humans
How do Th1 cells enhance antigen presentation by macrophages?
By increasing the expression of MHC class II molecules.
How do Th1 cells support CD8⁺ T-cell activation?
They activate CD8⁺ T-cells and promote CD40–CD40L interactions.
Th2 effectors
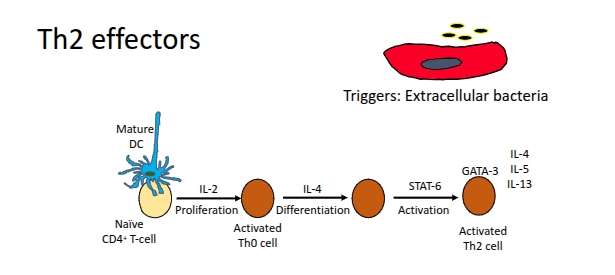
Which cytokines are secreted by Th2 cells?
IL-3, IL-4, IL-5, IL-6, IL-10, and IL-13
How do Th2 cells support B-cells directly?
They establish CD40–CD40L interactions with B-cells.
Which cytokines from Th2 cells induce class switching to IgG4?
IL-4 and IL-5
How do IL-4 and IL-13 affect macrophages?
They inhibit pro-inflammatory cytokine production, downregulate nitric oxide, and decrease Fcγ receptor expression
Which cytokine upregulates MHC class II on macrophages, dendritic cells, and B-cells?
IL-4
Which cytokines promote B-cell proliferation?
IL-4 and IL-13
What is the role of IL-5 in Th2 responses?
It promotes the growth, differentiation, and activation of eosinophils, especially in responses against parasites and helminth worms
Which cytokines promote mast-cell activation in helminth infections?
IL-3, IL-4, and IL-10.
What is the role of IL-10 in Th2 immunity?
It is anti-inflammatory and downregulates pro-inflammatory macrophages, IL-12 production, MHC class II, and B7 on macrophages and dendritic cells
Which transcription factors reciprocally regulate Th1 and Th2 differentiation?
T-Bet (Th1) and GATA-3 (Th2)
Which cytokine induces the expression of T-Bet?
IL-12
What does T-Bet promote and inhibit?
It promotes IFN-γ production but inhibits GATA-3, IL-4, and IL-5
Which cytokine induces the expression of GATA-3?
IL-4
What does GATA-3 promote and inhibit?
It induces IL-4 and IL-5 but inhibits IFN-γ
Th17 effectors
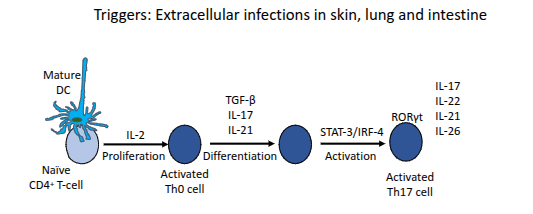
What are the main effector functions of Th17 cells?
They protect mucosal surfaces from pathogens resistant to Th1 and Th2 responses (e.g., Borrelia burgdorferi, Klebsiella pneumoniae, Pneumocystis carinii, Candida albicans); induce pro-inflammatory cytokines in non-haematopoietic cells; and can promote autoimmunity such as IBD, arthritis, and multiple sclerosis.
How does the Th phenotype change during malaria infection?
Early in infection, a Th1 response dominates, producing IFN to activate macrophages and induce B-cells to switch to IgG2a against intracellular parasites. Around ten days later, the parasite enters an extracellular phase, triggering a Th2 response with high IL-4, IL-10, and parasite-specific IgG1. Both the Th1 and Th2 phases are required to control the infection.
How do different Th phenotypes relate to immunopathology?
Th1 responses are associated with transplant rejection, Th2 responses with hypersensitivity, and Th17 responses with autoimmunity. These are generalisations, as many diseases involve mixed Th phenotypes occurring simultaneously.
Why does transplantation between genetically non-identical individuals often fail?
Because the recipient mounts an adaptive immune response against donor tissues. This was seen in WW2 burn patients, where transplanted skin necrosed and detached within 1–2 weeks. The reaction is driven largely by MHC differences and is known as rejection—an appropriate immune response to non-self tissue.
What happens in kidney transplantation when the donor and recipient are not compatible?
The recipient can experience an intense inflammatory reaction, including hyperacute rejection, caused by immune recognition of donor antigens—especially mismatched MHC molecules—leading to rapid graft failure.
What is an autologous graft?
A graft taken from one individual and transplanted back into the same individual.
What is a syngeneic graft?
A graft exchanged between two genetically identical individuals.
What is an allogeneic graft (allograft)?
A graft transplanted between two genetically different individuals of the same species.
What is a xenogeneic graft (xenograft)?
A graft transplanted between individuals of different species.
What are alloantigens?
Molecules in an allograft that are recognised as foreign by the recipient.
What are xenoantigens?
Molecules in a xenograft that are recognised as foreign by the recipient.
What does “alloreactive” mean?
Refers to lymphocytes and antibodies that react with alloantigens in an allograft.
What does “xenoreactive” mean?
Refers to lymphocytes and antibodies that react with xenoantigens in a xenograft
What type of immune cells infiltrate grafts during acute cellular rejection?
Lymphocytes and macrophages
Which T-cell subsets are involved in acute cellular rejection?
CD4⁺ and CD8⁺ T-cells specific for graft alloantigens.
Which cytokine-secreting CD4⁺ T-cell types contribute to graft rejection?
IFN-γ and TNFα-producing Th1 cells, and IL-17-producing Th17 cells
What is the role of macrophages and endothelial cells in acute rejection?
They become activated and contribute to inflammatory tissue damage within the graft.
What is hypersensitivity in immunology?
It is an inappropriate, overly active immune response to harmless environmental antigens such as pollen, foods, or animal dander. These substances induce sensitivity rather than protection, affecting around 1 in 6 people. Hypersensitivity diseases are mainly driven by either antibodies or T-cells and are classified into at least four types.
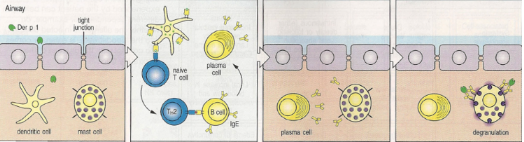
What is the mechanism of Type I hypersensitivity?
Allergens such as Der p1 are taken up by dendritic cells, leading to Th2 priming. Th2 cells induce class switching to IgE, which binds to FcεRI on mast cells. Upon re-exposure, mast cell degranulation releases histamine, prostaglandins, and leukotrienes, causing inflammation characterised by increased vascular permeability, oedema, and mucus secretion.
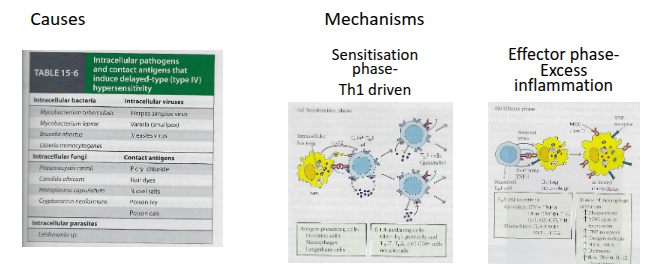
Type IV Delayed type Hypersensitivity (DTH)
What is autoimmunity?
It is the failure of tolerance mechanisms that normally prevent self-reactive lymphocytes from attacking the host. Autoimmune diseases affect 2–5% of the population, are most common in individuals aged 20–40, occur more frequently in women than men, and are typically chronic. In most cases, they involve an adaptive immune response directed against self-antigens.
How can polyclonal lymphocyte activation contribute to autoimmunity?
Agonists such as LPS can stimulate many lymphocytes at once, including self-reactive ones that escaped deletion. This leads to high levels of IgM not directed at specific pathogens (e.g., tetanus or flu), with some antibodies targeting self. Normally these self-reactive cells are cleared, but in conditions such as SLE and RA they persist and contribute to disease.
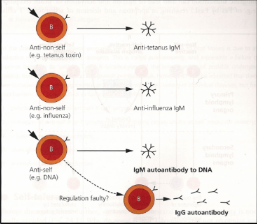
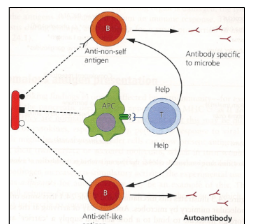
How does molecular mimicry lead to autoimmunity?
Some pathogens express antigens that resemble self-proteins. Because these antigens contain both self-like and foreign components, B-cells and T-cells recognise the non-self parts, allowing T-cells to provide help. If another B-cell recognises the self-like portion, it also receives this help, leading to an autoimmune response.
What mechanisms can trigger autoimmunity besides molecular mimicry?
Release of sequestered antigens: Normally hidden self-antigens (e.g., lens or sperm proteins) become exposed after tissue damage.
Anomalous antigen presentation: Upregulation of MHC class II in cells that do not usually express it enables inappropriate antigen presentation.
Anomalous cytokine production: Local inflammation can activate nearby lymphocytes with unrelated specificities, known as the bystander effect.