Ch. 9: Antimicrobial Chemotherapy
1/81
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
82 Terms
chemotherapy
anything given to a host to treat a disease
most chemotherapeutic agents are
antibiotics
synthetic antibiotics
comes from lab
ex: Salvarsan and prontosil (dye)
semi-synthetic antibiotic
modified true antibiotic
true antibiotic
(most) comes from living organism
Thomas & Bertheim
early research in chemotherapy
arsenical dyes
synthesized Atoxyl (→ blindness)
Atoxyl
arsenical that treats African sleeping sickness
caused by protist
Trypanosoma
Ehrlich and Hata
dyes and selective toxicity in bacteria
identified Salvarsan (abx)
syphillus
Salvarsan
treats Treponema pallidum (syphillis)
Domagk, Jacques and Trefouel
developed Prontosil dye (streptococcal & staphylococcal infections)
led to discovery of sulfonamides/sulfa
Gerhard Domagk
tried growing prontosil in a petri dish (en vitro) but it showed no activity. he tried it on his sick daughter (en vivo) and found that it did work
modified in living organism which made it work
prontosil is a pro drug which is inactive outside the body
Penicillin
first true antibiotic made by fungus penicillium
Ernest Duchesne
noticed fungi (penicillin) killing bacteria
Alexander Fleming
Staph petri dish with fungus contaminant - fungus (penicillin) is killing bacteria
couldnt isolate it
Florey, Chain, and Heatley
received Nobel Prize in 1945
isolated penicillin from penicillum; discovered therapeutic use
Waksman
first to identify streptomycin
treated M. tuberculosis
Actinomycetes
grow with filament
Streptomyces
bacteria; produces the most true antibiotics
why do bacteria kill other bacteria
competition
what antibiotics were discovered by 1953
chloramphenicol
terramycin
neomycin
tetracyclin
AI and antibiotics
2022:
AI yielded potential drugs Abaucin against Acinetobacter baumannii (microbe from ESCAPE, one of the worst pathogens, NARROW spectrum) and other drug-resistant bacteria
2023:
screened millions of compounds and tested 283 promising compounds
several were effective against MRSA
Abaucin
narrow spectrum
works against Acinetobacter baumanii
selective toxicity
ability of an antimicrobial agent to kill or harm the microorganism cells without harming the cells of the host
therapeutic index
ratio of toxic dose to therapeutic dose
higher index: better drug
drugs have a minimum concentration necessary to kill or inhibit growth of bacteria
aka how much drug you have to give to cause an outcome to microbe
broad vs narrow spectrum antibiotics
narrow spectrum can only kill a few microbes
obligately parasitic bacteria: atypical
no antibiotics for viral infections
how is effectiveness of antimicrobial drugs expressed
effect of an agent may vary
expressed in 2 ways
minimal inhibitory concentration (MIC)
minimum lethal concentration (MLC)
minimal inhibitory concentration (MIC)
static
drug inhibits microbe from continuing replication
minimum lethal concentration (MLC)
cidal
destruction of the cell, can kill microbe - doesn’t wait for immune system
need this if immunocompromised
lytic: breaks it apart
do not want to use bacteriolytic abx with gram-negatives because lipid A is an endotoxin
ways to determine level of antimicrobial activity
dilution susceptibility tests for MIC
drug inhibits microbe from further replication
disk diffusion test (Kirby Bauer)
zone of inhibition: clear area around the disc has no bacterial growth - sensitive to abx
bigger zone doesnt mean it’s better
the Etest
strip test
main modes of action of antimicrobial drugs
inhibitors of cell wall synthesis
protein synthesis inhibitors
metabolic antagonists
nucleic acid synthesis inhibition
inhibitors of cell wall synthesis
most specific to bacteria (peptidoglycan cell walls)
no inhibitor of cell membrane because cell membrane between bacteria and eukaryotes are very similar
Mycoplasma resistant to these abx
antibiotics are most effective when bacteria are actively dividing (new cell walls being synthesized)
inhibitors of cell wall synthesis - PENICILLINS
first identified true abx from fungus Penicillium - penicillin V and G
semisynth alternatives (ampicillin)
bactericidal (destroy)
β-lactam ring - four sided ring
1-5% of adults in US are allergic
β-lactam ring
recognized by penicillin binding proteins
inhibits transpeptidation in bacteria from making peptide bonds
β-lactamase/penicillinase (specifically penicillin)
what happens if the cell wall is destroyed
there is no protection against osmotic pressure, causing the cell to lyse
β-lactamase/penicillinase
enzymes that bacteria contain to break down the β-lactam ring and destroy penicillin
penicillin is unable to block transpeptidation, allowing bacteria to survive
Penicillin V and G
against gram-positive only
narrow spectrum
what spectrum are semisynthetic penicillins
broad
semisynthetic penicillins
Ampicillin
Carbenicillin
Piperacillin
Nafcillin
made more soluble to be able to get to peptidoglycan
Ampicillin
works against Gram-negative H. influenzae, Salmonella spp. and S. dysenteriae
inhibitors of cell wall synthesis - CEPHALOSPORINS
bactericidal, broad spectrum
true antibiotic made by fungus (mold) Acremonium (previously Cephalosporium)
can be used for those allergic to penicillin
β-lactam ring
has resistant bacteria
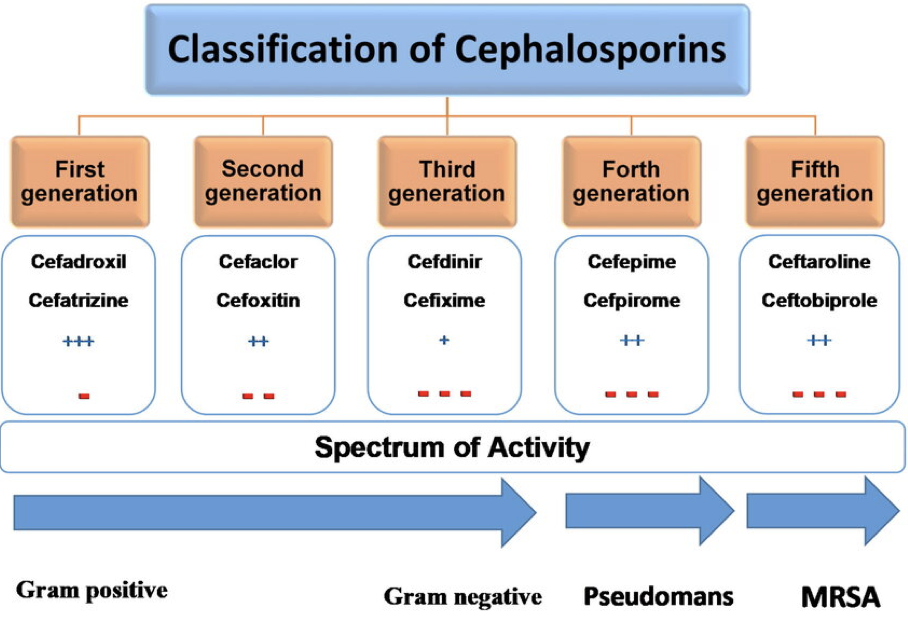
resistant bacteria against Cephalosporins
N. gonorrhoeae and Enterobactericeae HAI
inhibitors of cell wall synthesis - CARBAPENEM and other β-lactam
semi-synthetic
combination of penicillin and cephalosporin
broad spectrum
β-lactam
type of penem = penicillin and cephalosporin hybrid drugs
penem
modified penicillins with
→ Sulfur replaced by carbon
→ A double bond added to the ring
pentane → pentene
what antibiotics have β-lactam rings
Penicillin
cephalosporins
carbapenem
inhibit transpeptidation from making peptide bonds → destroys bacteria
inhibitors of cell wall synthesis - VANCOMYCIN
bacterialcidal, narrow-spectrum
gram-positive only: Staphylococcus, Clostridium, Bacillus, Streptococcus, Enterococcus
Clostridium + Bacillus: sporeformers
turns into spore when exposed to abx and will not get killed
true antibiotic produced by bacterium Streptomyces orientalis
inhibits transpeptidation by not allowing removal of terminal alanine
treats antibiotic-resistant staphylococcal and enterococcal infections
previously considered “drug of last resort” against MRSA
vancomycin resistant enterococcus (VRE)
gram-positive
no beta-lactam ring
entero means?
intestines
protein synthesis inhibitors
multitude of steps within protein synthesis
generally associated w/ ribosomes or steps that lead to process of translation within ribosomes
side effects associated bc of mitochondria also being 70s
many antibiotics bind specifically to bacterial ribosome
bacterial ribosome
small subunit: 30s
16s rRNA: aligns ribosome w/ shine-dalgarno on mRNA and decodes mRNA during translation
large subunit: 50s
23s rRNA: peptidyl transferase activity; forms peptide bonds between AA
70s ribosome
the subunits are made of rRNA or protein
30s subunit
reads mRNA
tRNA anticodon recognizes codon
30s ensures correct codon-anticodon pairing
drugs that target 30s:
aminoglycosides: mRNA misreading
tetracyclines: blocks tRNA from binding at A site
50s subunit
tRNA - APE site
tRNA brings AA to recognized codon and allows peptide bonds to form between 2 AA
eukaryotic ribosome
small subunit: 40s
large subunit: 60s
80s ribosome
steps that antibiotics inhibit in protein synthesis
aminoacyl-tRNA binding
peptide bond formation
mRNA reading
translocation
protein synthesis inhibitors - AMINOGLYCOSIDES
bacteriocidal, broad-spectrum
binds to 30s subunit of ribosome
true antibiotics:
streptomycin, kanamycin, neomycin by bacteria Streptomyces spp.
Gentamicin by bacteria Micromonospora spp.
all other abx are semisynthetic
can be toxic and cause renal damage
Gentamicin
made by bacteria Micromonospora spp.
used for aerobic gram-negative Proteus, Escherichia, Klebsiella, and Serratia
protein synthesis inhibitors - TETRACYCLINES
4 rings
bacteriostatic, broad spectrum
binds to 30s subunit of ribosome
treats intracellular pathogens rickettsias, chlamydiae, and mycoplasmas
true antibiotics, Oxytetracycline and chloretetracycline prod by Streptomyces spp.
others are semisynthetic (ex: doxycycline)
side effects: black teeth (chelating of calcium) and liver toxicity
not Rx to pregnant women and young children (< 8 yrs)
protein synthesis inhibitors - MACROLIDES
large bacteriostatic, broad spectrum; contains lactone ring
binds to 50s subunit
treats against gram-positive, mycoplasma spp., and some gram-negative
erythromycin is a true abx (bacteria Saccharopolyspora), all others are semisynthetic
Azithromycin - chlamydia (atypical) “Z-pack”
most common Rx along with amoxicillin
used for pt’s allergic to penicillin and cephalosporin
protein synthesis inhibitors - LINCOSAMIDES
large bacteriostatic, broad psectrum
binds to 50s subunit
treats against gram-positive cocci, some gram-neg anaerobes, and β-lactamase producers
true abx produced by Streptomyces spp.
disrupts colon’s microbiota, supporting growth of C. diff (gram-pos rod spore former)
treats CA-MRSA
clindamycin
problem with prescribing lincosamides
disrupts colon’s microbiota, supporting growth of C. diff (gram-pos rod spore former)
C. diff leads to pseudomembranous colitis → no absorption of nutrients, diarrhea, hard to kill
Lincosamides are a last resort because CDI is problematic and requires a fecal transplant to treat
should be reserved for serious infx where less toxic antimicrobial agents are innappropriate
do not use in pt’s with nonbacterial infections
protein synthesis inhibitors - OXAZOLIDINONES
bacteriostatic, broad spectrum
may be bactericidal for some bacteria
binds to 50s subunit (specifically 23s rRNA) → no 70S formation
synthetic
linezolid introduced in 21st century
treats MRSA (methicillin), VRE (vancomycin), penicillin-resistant S. pneumoniae, and some gram-pos anaerobes
very few side effects, only used IN hospitals
protein synthesis inhibitors - CHLORAMPHENICOL
bacteriostatic, first broad-spectrum
binds to 50s
against most gram-pos, many gram negative anaerobes, and rickettsias
true abx produced by streptomyces venezuelae
toxic with numerous side effects
aplastic anemia, leukemia, neurotoxin reactions
grey baby syndrome
inhibits mitochondrial protein synthesis
not allowed in food-producing animals
metabolic antagonists
binds to enzymes that we don’t have, inhibits pathways that aren’t used
acts as antimetabolites bc they look like the real version
ex: PABA and SFA
both fighting to bind to active site, if there is more of SFA, it’s more likely to bind than PABA, though PABA still has a chance
enzyme is only looking for the left side and binds to SFA (doesn’t notice difference)
structural analogs
bacteriostatic, broad spectrum
binding of substrate to enzyme isn’t covalent, can detach
folic acid is required for production of
DNA
metabolic antagonists - SULFONAMIDES/SULFA DRUGS
bacteriostatic, broad spectrum
synthetic
selectively toxic d/t competitive inhibition
works against some protozoa (unicell euk)
inhibits making of folic acid → pathways with no function
folic acid is precursor for purines (A and G), methionine, and ATP
without folic acid: no DNA, RNA, proteins, or energy
sulfonamides are a p-aminobenzoic acid (PABA) analog
inhibits dihydropteroate synthase (1st step for folic acid production)
metabolic antagonists - TRIMETHOPRIM
bacteriostatic, broad spectrum
synthetic
inhibits dihydrofolate reductase (2nd step for folic acid production)
when combined with sulfa drugs, it is more effective treatment, inhibiting the 1st and 2nd steps
synergism, works better together
forms Bactrim
side effects: abd pain + photosensitivity
nucleic acid synthesis inhibition
least specific with most side effects bc RNA and DNA polymerase look alike and are hard to target
can inhibit transcription (RNA) and replication (DNA)
variety of mechanisms
most commonly
inhibit RNA polymerase (rifamycin)
inhibit topoisomerases (fluoroquinolone)
not as selectively toxic as other abx bc bacteria and eukaryotes do not differ greatly in the way they synthesize nucleic acids
small toxicity index
topoisomerases
unwind and separate DNA, prevents overcoiling and getting stuck
nucleic acid synthesis inhibition - FLUOROQUINOLONES
bactericidal, broad spectrum
synthetic
highly effective against enteric bacteria
inhibit bacterial DNA-gyrase and topoisomerase IV (type II topoisomerases)
Norfloxacin first approved for humans in 1986
currently 9 approved for humans
ciprofloxacin (Cipro)
tendonitis and tendon rupture
nucleic acid synthesis inhibition - RIFAMYCINS
bactericidal, broad spectrum
Rifampin is most used member (semisynthetic)
blocks transcription by binding to RNA polymerase (β-subunit)
used:
in multidrug treatment of TB & other mycobacterial infections (leprae)
Can be used prior to disease - for prophylaxis of close contacts of patients with meningococcal meningitis or (bacteria) Haemophilus influenzae type B meningitis
may cause red sweat or urine
inhibit RNA polymerase
antiviral drugs
drug development is slow because it is hard to identifiy since viruses use enzymes from our cells
drugs currently used inhibit virus-specific enzymes and life cycle processes
difficult to treat viral infections w/ chemotherapeutic agents bc viruses use metabolic machinery of their hosts which limits potential points of attack
antiviral drugs for influenza
influenza is segmented RNA virus
Tamiflu (oseltamivir)
neuraminidase inhibitor → no virion release → halted viral replication
Not a cure for influenza, but shortens course of illness
Inhibits virus from binding to cell and replicating
Xofluza (marboxil baloxavir)
blocks transcription of DNA → RNA
2018
there are viruses resistant to both drugs
antiviral drugs for Herpesviridae
Herpesviridae are DNA viruses
use their own enzymes to phosphorylate nucleosides → nucleotides
Analogs that inhibit virus replication
Acyclovir and vidarabine
HIV
mutates quickly
can’t be cured
low viral load (hard to identify)
cocktail of multiple anti-HIV drugs targets the virus from multiple angles, inhibiting the angles from mutating
anti-HIV drugs
Nucleoside reverse transcriptase inhibitors (NRTIs) produce faulty DNA
a. includes AZT
Nonnucleoside reverse transcriptase inhibitors (NNRTIs) prevent HIV DNA synthesis
Protease inhibitors (PIs) mimic peptide bond that is normally attacked by the protease
Integrase inhibitors prevent HIV genome incorporation
Fusion inhibitors prevent HIV entry into cells
Most successful are drug cocktails to curtail resistance (HAART)
Multiple drugs work together to inhibit
Preexposure prophylaxis (PrEP) – two NRTIs daily
vaccines
antiviral drugs for Hep C
RNA virus (mutates faster than DNA because RNA polymerase more error prone than DNA viruses)
possible opportunistic infection of HIV
cases of acute hep C have increased in US since 2010
Interferon-α (cytokine) and ribavirin (antiviral) was first used
nucleotide analog
These drugs given together cause too many side effects (depression)
Since 2014, combination drugs
sofosbuvir-velpatasvir (Epclusa)
sofosbuvir-ledipasvir (Harvoni)
antifungal drugs
fewer effective agents
many have low therapeutic index and are toxic
d/t being eukaryotes, identifying something unique to them is difficult
Easier to treat superficial mycoses than systemic
Many are fungistatic
Fungi prefer to grow in cooler temps
treating superficial mycoses
nystatin (from Streptomyces) – used to control superficial candidiasis
Thrush from Candida albicans
treating systemic mycoses
harder to treat
polyenes and azoles – block cell membrane synthesis (ergosterol - cell membrane of fungi)
amphotericin B (from Streptomyces) – binds ergosterol in membranes, highly toxic
5-flucytosine – uracil analog, disrupts RNA function
fluconazole – low side effects, used prophylactically for immunocompromised patients
Echinocandins
inhibit β-1,3-D-glucan (building block of fungal cell wall) synthase
similar to peptidoglycan inhibitors
Some Candida and Aspergillus spp. have β-1,3-D-glucan cell wall
Pneumocystis jiroveci has cholesterol instead of ergosterol
uses trimethoprim (sulfa drug) and sulfisoxazole instead
antiprotozoal drugs (eukaryotes)
mechanism not precisely known - maybe nucleic acid or some metabolic process
Chloroquine - Plasmodium (malaria)
Metronidazole
reduced to RNS (reactive nitrogen species) by Entamoeba (dysentery) and Trichomonas (vaginal infection)
combination therapy of pyrimethamine, dapsone, sulfadiazine, folinic acid – Toxoplasma gondii (abortion)
pyrimethamine and dapsone similar to trimethoprim
inhibit folic acid; purines; A/G; ATP
E. histolytica: dysentery
T. gondii: toxoplasmosis
P. falciparum: malaria
T. brucei: african sleeping sickness
Bacteria: T. pallidum: syphilis
drug effectiveness
need to know:
how the drug arrives at the site of infection
pathogen is susceptible
drug level concentration exceeds pathogen’s MIC
cannot go lower than the MIC
drug resistance
microbes in abscess or biofilms
resistance mutants
mechanisms for drug resistance
intrinsic or acquired (potentially mutation)
mechanisms include:
modify the abx target
enzymes are specific
enzymes degrade or alter abx
β-lactamase
decrease abx concentration inside cell
efflux pumps
use an alternate pathway or increase target metabolite production
change how much metabolite is present; PABA and sulfa
biofilm formation
acquisition of resistance genes thru HGT
overcoming drug resistance
give drug in appropriate concentrations
give 2 or more at same time (cocktails: less likely for microbe mutation)
use drugs only when necessary
possible future solutions:
phages - can use in plants
lytic and lysogenic