BIPN 105 Sciatic Nerve and NMJ
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
49 Terms
What did we observe in the sciatic nerve lab?
Axon function, we cut cell bodies and terminals to just have axons
Membrane potential (Vm)
sodium potassium pumps set up and maintain Vm
maintain more potassium inside, low sodium inside
Determined by the Goldman Equation
Goldman Equation
potassium permeability is much higher than sodium due to significantly more leak channels being available
at 37 degrees C
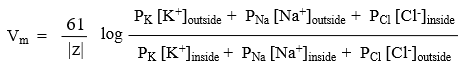
Equilibrium Potential
use nernst equation
EK=-90mV
ENa=+61mV
ECl=-70mV
when the chemical potential is equal to the electrical potential
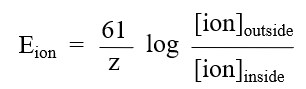
Action Potential
an all or none response to depolarization
Action potential Phases
are generated by voltage-gated ion channels activity dependent on the Vm
Depolarization, Repolarization, Hyperpolarization
Depolarization
Triggered by sodium channel activation as a result of depolarization, the pore activates creating a pathway for sodium influx. The electrochemical gradient is driven by the very positive equilibrium potential of sodium. This facilitates sodium entry further depolarizing the membrane. This triggers a cascade of sodium channel activation until all channels are engaged
Repolarization
The time dependent inactivation gate of the sodium channels closes blocking the pore and potassium channels activate opening their pore as a result of depolarization, allowing the potassium to exit resulting in a rapid repolarization since the equilibrium potential of potassium is highly negative
Hyperpolarization
when you have excess potassium permeability and excess potassium current leading to the membrane potential to go past Vrest.
Return to Vrest
Potassium channels deactivate as a result of repolarization and sodium channels remove their inactivation gate as a result of repolarization resulting in the deactivation of sodium channels. Then the membrane potential returns to rest as a result of leak channels being the only open channels
Refractory Period
how long a neuron must wait before it can fire a second AP
Absolute Refractory
No matter the strength of a second stimulus, there is no resulting AP due to the sodium channels being inactivated
relative refractory
when a larger second stimulus could yield a second action potential due to the hyperpolarization phase and high permeability of potassium
Lab assumption for refractory
The stimulus is large enough that any neuron in RRP will fire a second action potential and if the neuron does not fire a second action potential, it is in its absolute refractory period.
Conduction velocity
how fast an action potential propagates down an axon
What increases conduction velocity?
larger diameter
greater myelination
fiber types
myelination
a lipid bilayer wrapped around the axon that acts as insulation so passively spreading depolarization doesn’t drop below VT
Saltatory Conduction
AP jumps from one node of ranvier to another and only regenerates AP at the nodes resulting in much faster AP spread
Fiber types
A fibers - large myelinated, 20-100 m/s
C fibers- small unmyelinated- 1-2 m/s
Compound Nerves
bundles of different types of axons
CAP
summation of individual APs; this is what is measured from the sciatic nerve by extracellular recording
not all or none
max CAP is when all neuron types have been recruited
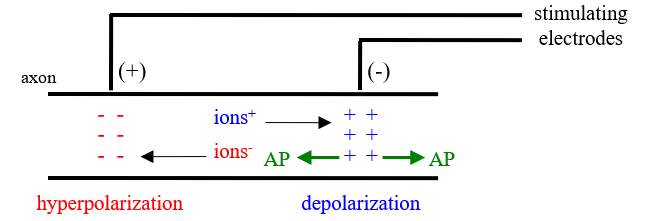
Stimulus
using an electrical stimulator
enough positive ions accumulate to reach VT starting the AP at the negative
AP goes both ways
at the positive stimulating electrode there is hyperpolarization,
Extracellular Recording
Recording electrodes only measure the voltage outside the neuron near the electrode- DOESNT measure membrane potential
intracellluar recording would be for a single neuron, extracellular for the nerve
Record the positive and then subtract the outside voltage of the negative recording electrode, if the AP is too far it will not be recorded by either axon
the max diff in - and + gives a large positive deflection
negative monopolar- - recording is subtracted from the ground
Sciatic Nerve
mainly A fibers, record with - first by convention to yield a positive deflection, using an AC couple
Conduction velocity in the Lab
position E- Position D/ latency at E- latency at D
refractory period lab setting
double pulse with decreasing interpulse interval , maintain delay and duration
CAP2/CAP1 decreases= less neurons recruited and reaching Vt, IN ARP
Stimulus polarity results explained
with the negative stimulus, the positive stimuluating electrode serves as the negative and the negative stimulating electrode serves as the positive therefore there is hyperpolarization that the AP must pass through resulting in a lowered stimulus potential and thus less neurons reaching threshold and a lower cap
this also increases the distance from the stimulating electrode resulting in an increased latency
Strength-Duration
threshold depends on amplitudes and duration of the stimulus.
decreased duration requires a greater stimulus amplitude to yield a criterion sized CAP
Rheobase
the minimum voltage to elicit a criterion sized CAP at infinitely long durations
chronaxie
a measure of nerve excitability, smaller chronaxie means the nerve is more excitable . Duration of the stimulus needed when amplitude is twice the rheobase
Neuromuscular Junction Anatomy
vesicles
300,000/ terminal
10,000 ACh/ Vesicle
synaptic cleft
30 nm wide
filled with basal lamina
receptors
nmAChR
respond to ACh- cholinergic
sensitive to nicotine- nicotinic
on skeletal muscle
Vrest is close to Ek
Fk= very small
FNa= very large
therefore, despite being a mixed ion channel, mainly sodium enters for depolarization
nmAChR
ligand dependent ion channel
requires 2 ACh to activate
nonspecific mixed ion channel
Nerve AP→ MAP
Aα motorneuron→AP→ Presynaptic Terminal→ activates P/Q Ca++ Channels→Ca++ influx
vesicle exocytosis (~300)→ACh release→ ACh diffuses across the cleft→binds to nmAChR
nmAChR activation→ postsynaptic depolarization→ EPP is graded (increase ACh increases EPP)
MAP?
small ACh?→ EPP too small to reach VT, no MAP
enough ACh→EPP> VT→ get MAP!
1 NMJ per muscle, in the middle of the muscle cell
What must EPP do for a MAP
the EPP starts in the center of the muscle cell and must activate the sodium channels for the MAP
Termination
stop exocytosis
ca++ diffuses away from the vesicles
[Ca++]in—ATPase Pumps—>[Ca++] out
ACh disassociates from nmAChR→deactivates→ EPP dissipates
ACh removed due to the fact that as long as it is in the cleft it will activate receptors
diffuses away and enzymatic breakdown in cleft ( ACh—AChE→ choline+acetate
ACh is removed in <1 ms
Recycling
ACh broken down into Choline by AChE
Choline uptake into the presynaptic terminal by the choline- Na+ cotransporter
the Na+ concentration gradient from the Na+-K+ pumps allows this
choline +AcCoA—ChAT(choline acetyltransferase)—>ACh+CoA→ put in vesicles by VAChT
Fatigue
after high frequency stimulation
store 300,000 vesicles/terminal, release 300 vesicles/ AP
deplete ACh/ vesicles resulting in fatigue
Recovery requires the formation of new ACh/ vesicles which may take seconds to minutes
Synaptic Delay
delay between presynaptic depolarization and postsynaptic depolarization
around 0.5 ms due to
release of NT ~0.3ms
longest step
if you slow this step, you increase synaptic delay
diffusion across the cleft ~0.05ms
fastest step
nmAChR activation ~0.15 ms
IN LAB, the delay is from presynaptic depolarization to MAP recording, not the beginning of the EPP. This adds the time that sodium enters the cell, time the EPP needs to reach threshold, the time to fire an action potential and the time for the AP to spread throughout the muscle membrane to be detected by recording
Synaptic Delay calculation in Lab
MAP latency- conduction time
conduction time: distance from - stimulating electrode and negative muscle recording electrode divided by the CV of the nerve
MAP latency- onset of the MAP- onset of the stimulus
Facilitation
may occur if multiple stimuli are used
does NOT change the AP of the motorneuron, these are all or nothing
with a wide interval , there is no facilitation
with a short interval
residual intracellular Ca++ near presynaptic terminal membrane increases P/Q channel activation, sensitive to calcium channels so they activate better increasing calcium influx
if the stimulus occurs very quickly exocytosis doesn’t get rid of all the calcium from the previous
increased Ca++ influx caused increase ACh release and therefore a greater EPP every time due to more nicotinic receptor activation
Nerve vs SKM comparison
Vrest
Aα motorneuron: -70 mV
SKM- -90mV
Vthreshold
Aα motorneuron: -50 mV
SKM- -50mV
ARP
Aα motorneuron: 1 ms
SKM: 3-4 ms
CV
Aα motorneuron: 20-100 m/s
SKM: 1 m/s, no myelination
Other NT/ Receptors
Glutamate
glutaminergic receptors
CNS excitatory
GABA
GABAnergic
CNS inhibitory
Norepinephrine
adrenergic
sympathetic→postganglionic neuron (released)→tissue CNS receives
ACh
cholinergic
can be blocked with atropine
nicotinic
NMJ (nmAChR)
ANS+ CNS nNAChR
muscarinic
parasympathetic: postganglionic neuron releases it onto CNS tissue
Myasthenia Gravis (Symptoms, Cause, Diagnosis, Treatment)
Symptoms
muscle weakness/ fatigue with repetitive use
starts in small cranial muscles
extraoccular- double vision when reading for a while
throat- swallowing problems after a while
progresses to larger muscles
arms, legs, breathing?
Cause
autoimmune attack on nMAChR
Diagnosis
have the patient do repetitive contractions until fatigue starts
squeeze a ball, hold arms above head, etc
inject edrophonium ( short acting AChE inhibitor)
30 seconds→ decrease fatigue
10 minutes→ fatigue returns
Treatment
long acting AChE inhibitor
anti-immune therapy
Drugs applied in lab to?
middle of the muscle
For fatigue and recovery
we used the grass stimulator with no delay at repetitive stimulation for 4 minutes
Why is stimulus artifact smaller in the MAP than the CAP
we used two grounds
there is a longer distance between stimulating and recording electrodes resulting in passive spread dissipating
the artifact hits both recording electrodes at the same time and thus is subtracted from the recording- MAP is bipolar
Normal synaptic delay range in lab
1-3 ms
why would a CV be too low?
nerve is damaged
CAP didn’t record right, they are too far apart