Chemistry Final Review 2024
1/209
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
210 Terms

What is a graduated cylinder used for?
Measure volume of liquids (most accurate)

What is a beaker used for?
Hold liquids (volume)

What is a flask used for?
Measure, hold, mix etc. liquids (volume)
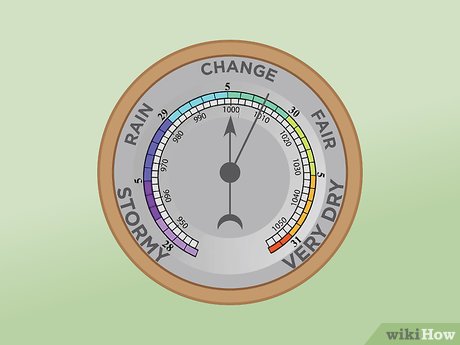
What is a barometer used for?
Measure air pressure

What is a manometer used for?
Measure pressure of fluid or gas

What is a calorimeter used for?
Measure heat of a reaction
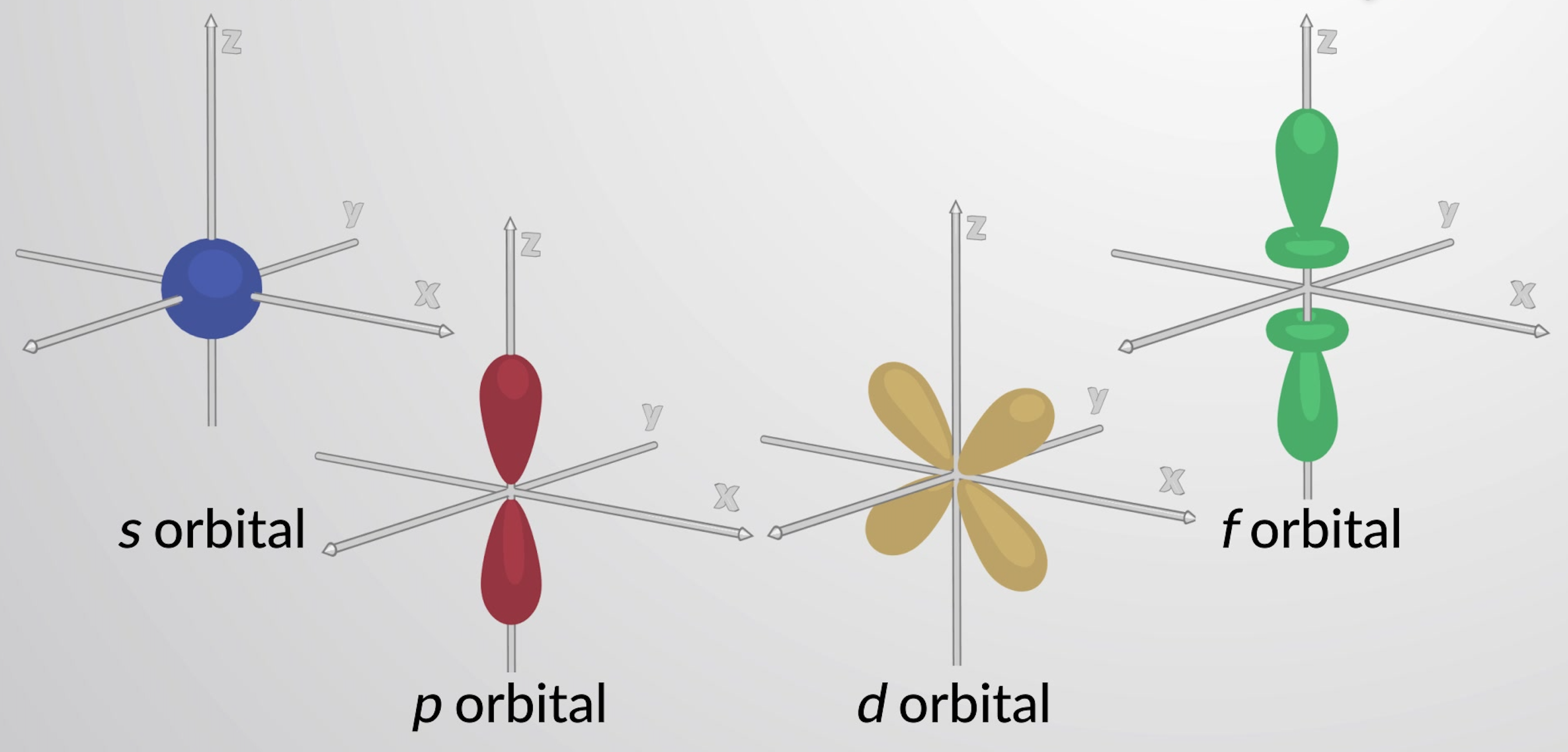
What is the shape of the s-orbital?
Sphere
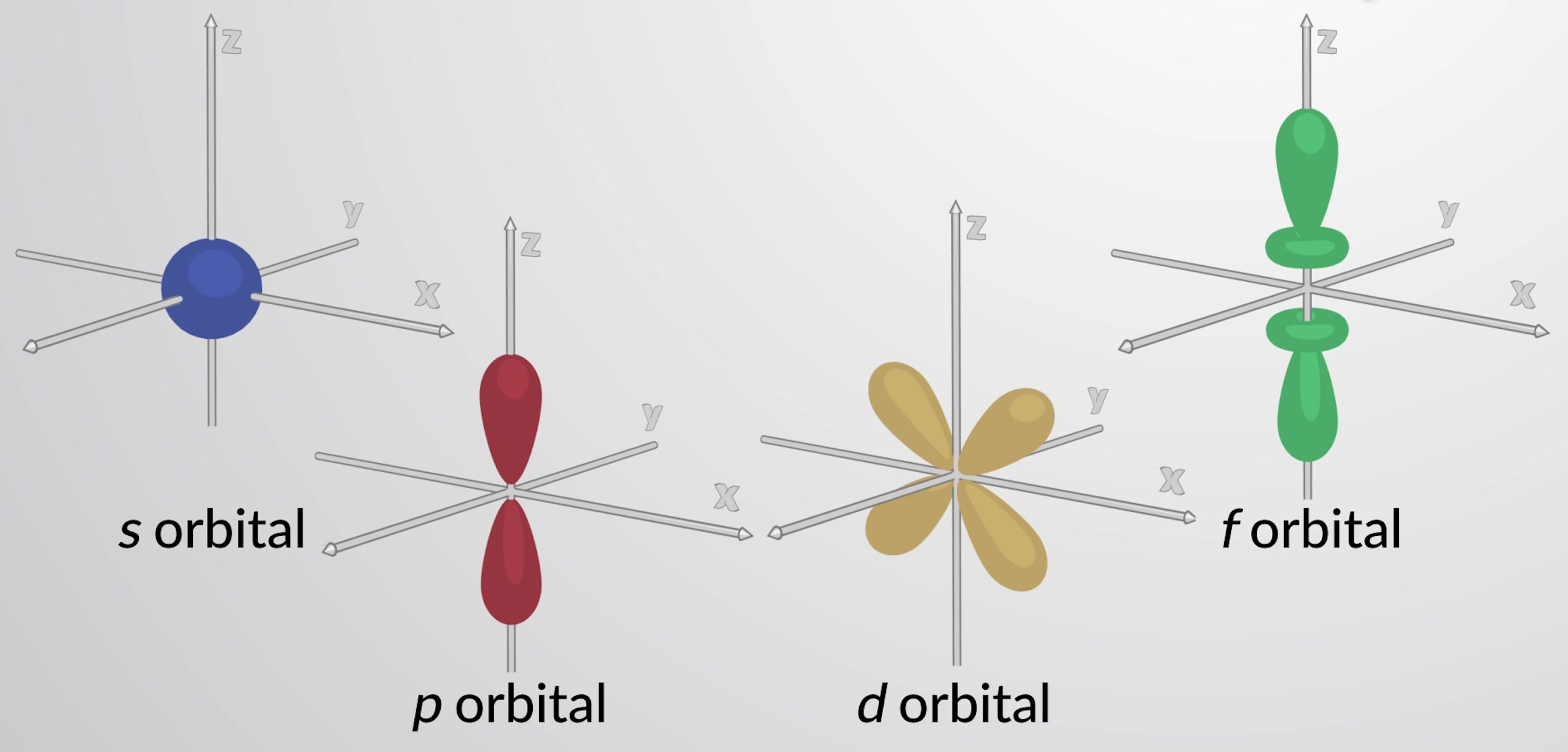
What is the shape of the p-orbital?
Dumbbell
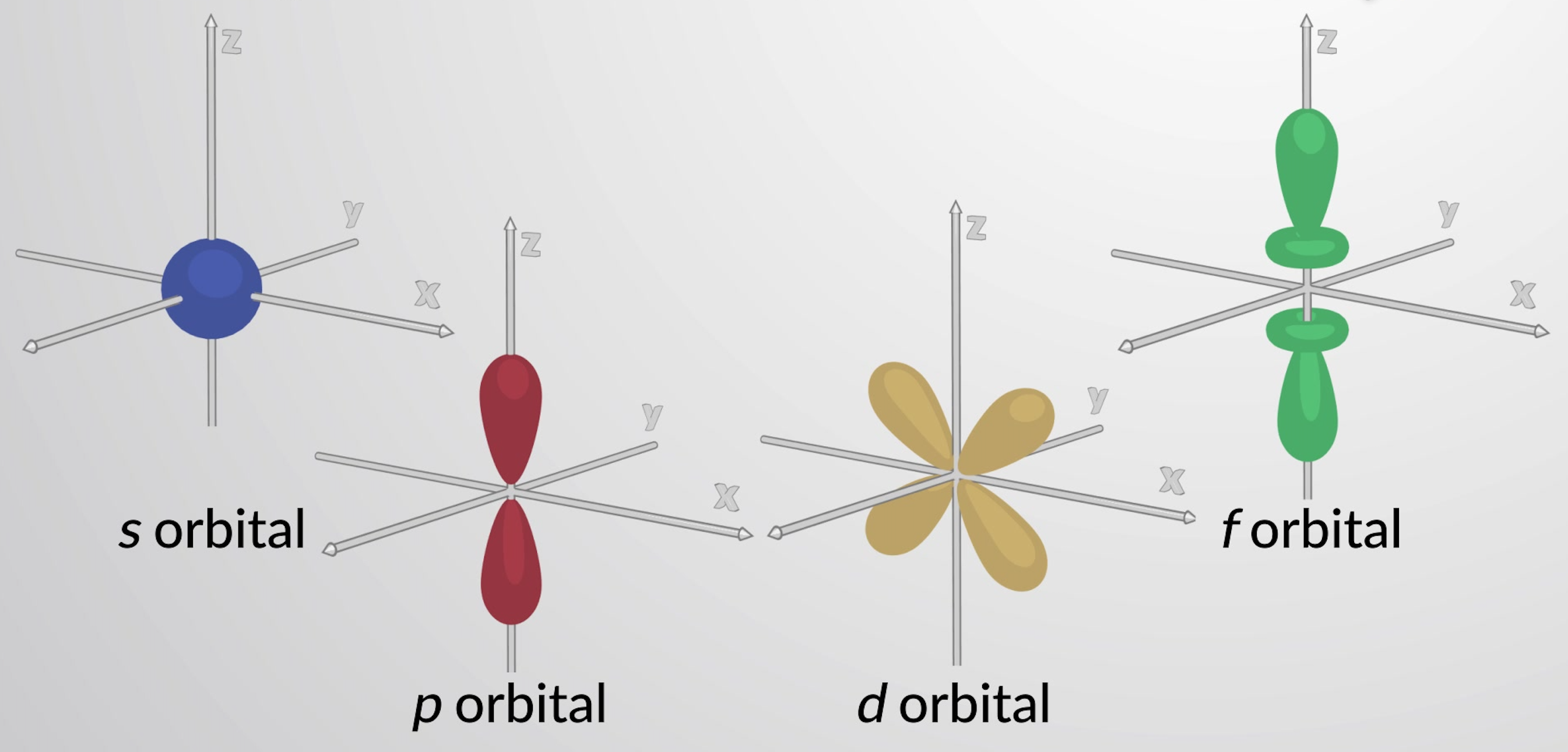
What is the shape of the d-orbital?
Four-leaf clover; dumbbell with a donut
What is Pauli Exclusion principle?
-Only 2 electrons per orbital
- Must have opposite spins
-No two electrons can have the same 4 quantum numbers
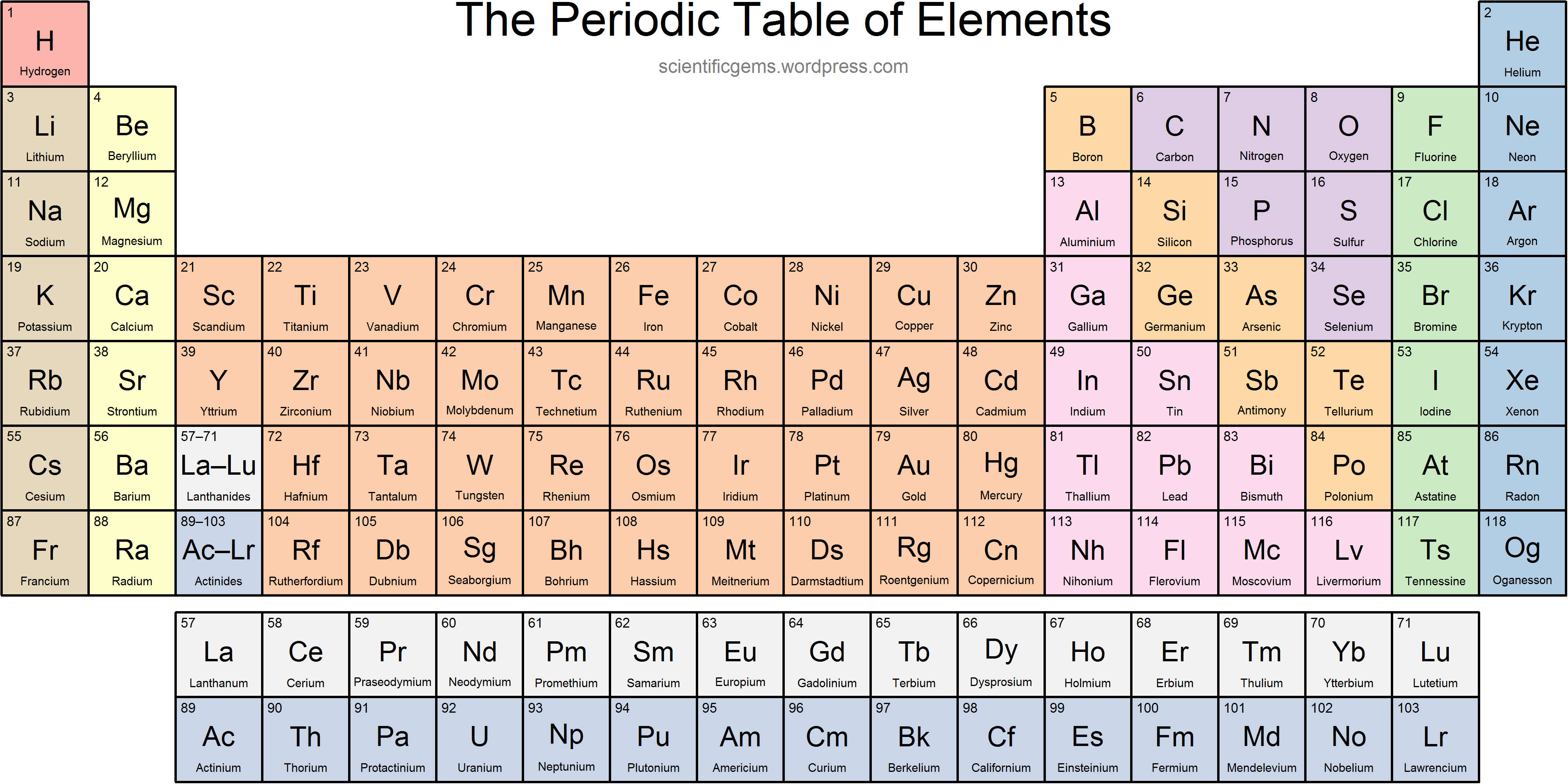
What is the name of Group 2?
Alkaline Earth Metals
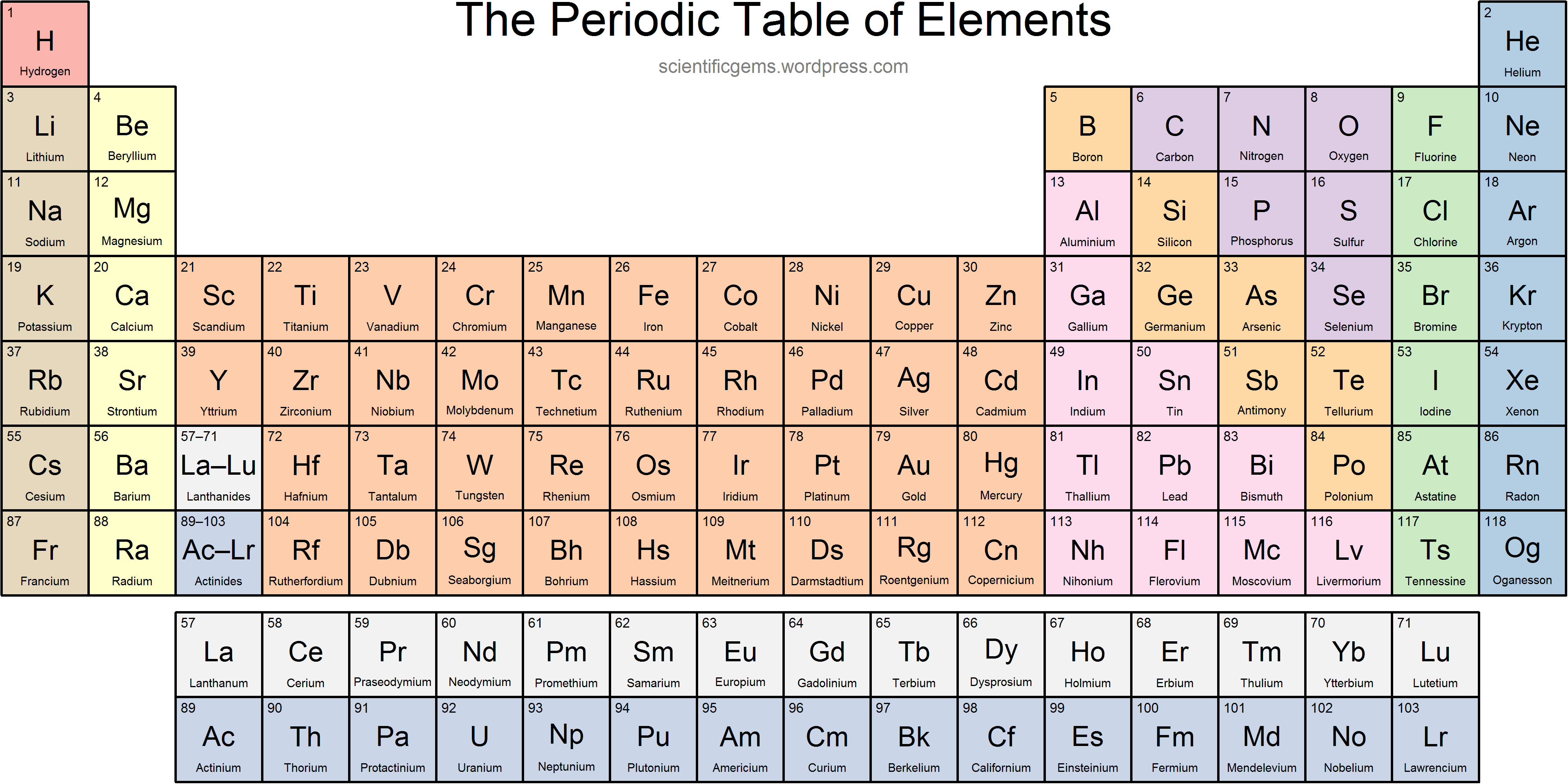
What is the name of Group 17?
Halogens
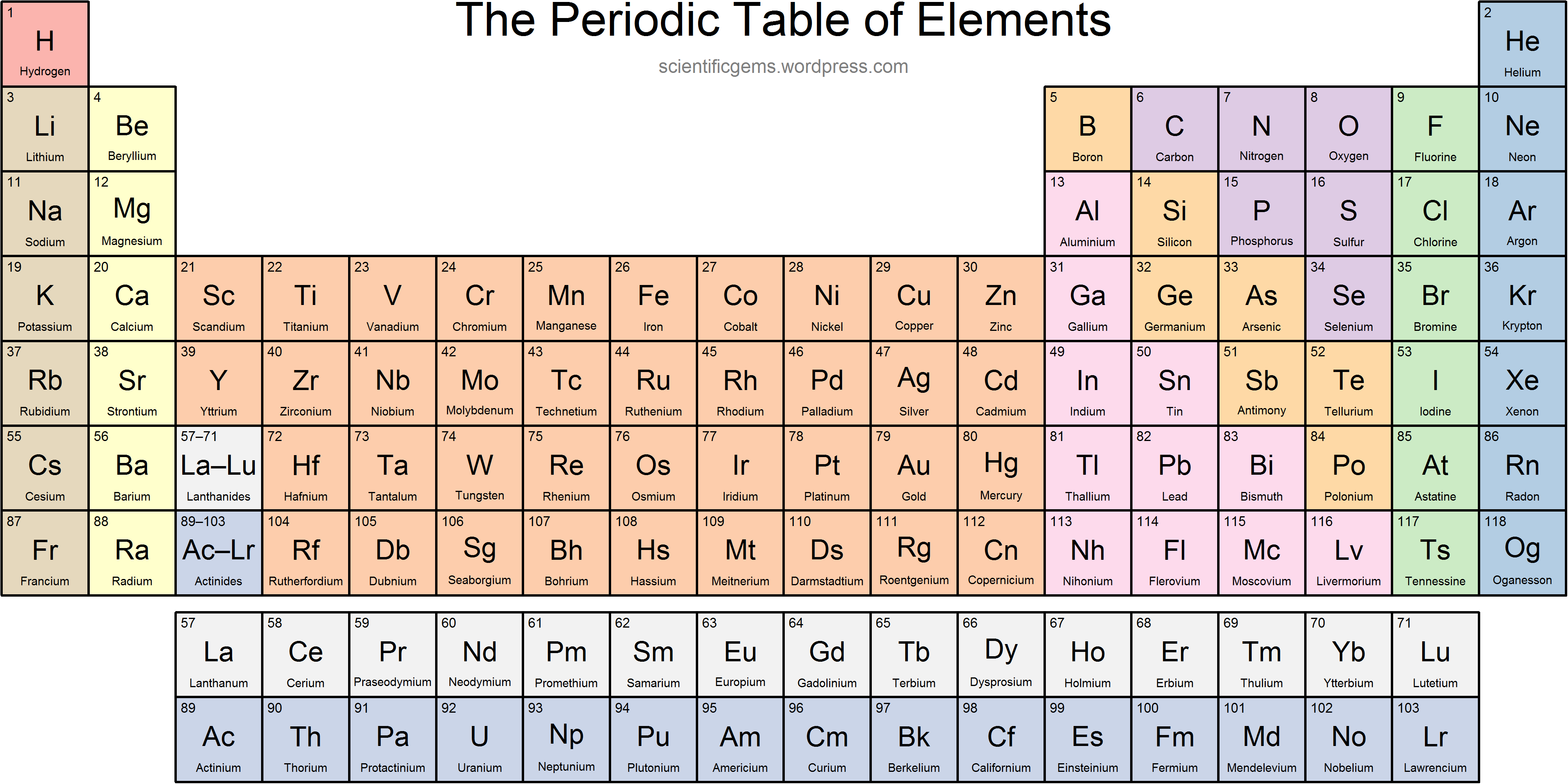
What charge does Group 17 form?
-1
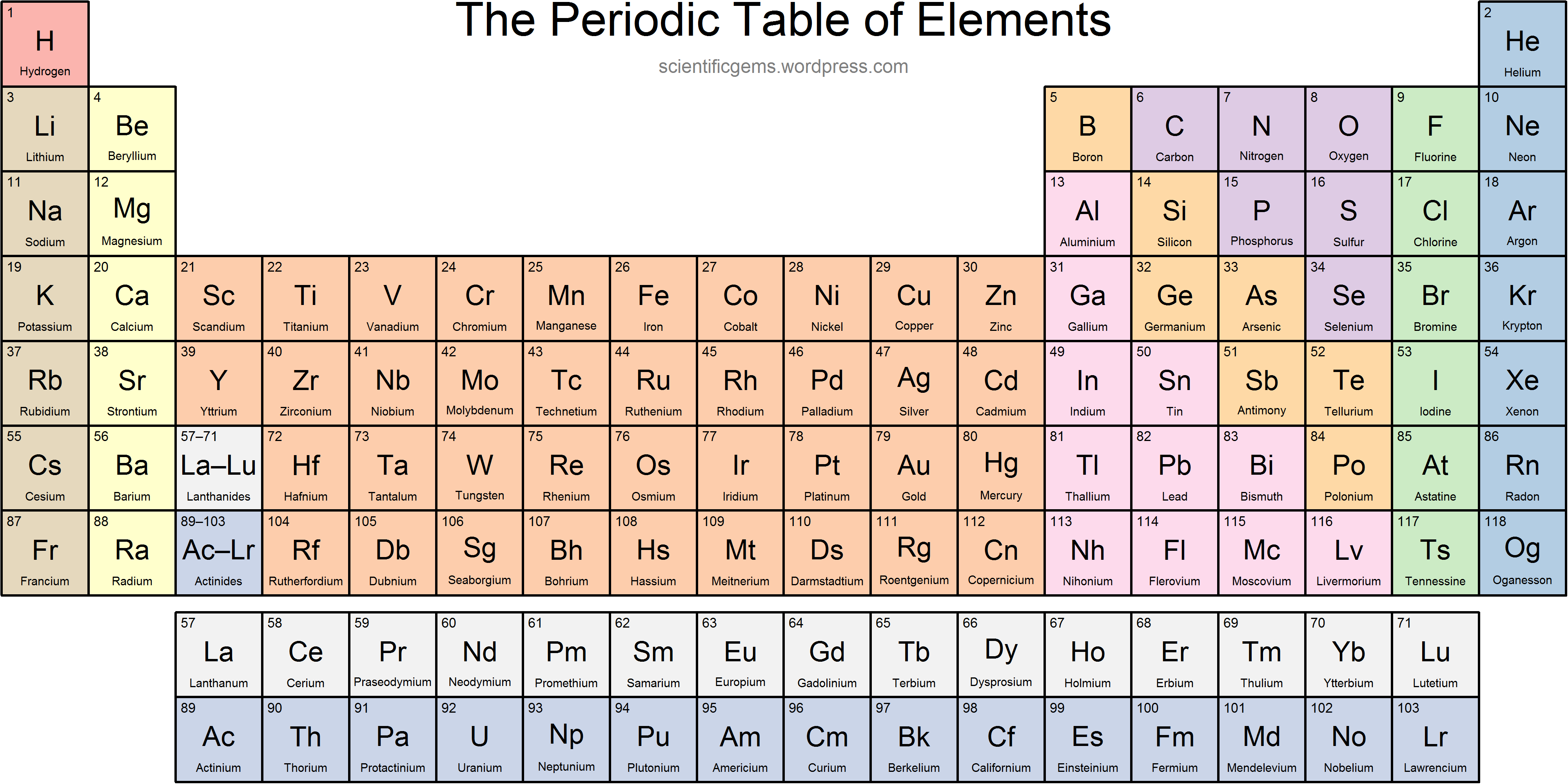
Where are the lanthanides located on the periodic table?
F-block; Elements 57-71
What are properties of metals?
-Shiny/Luster
-Solid at 25C (expect Hg, which is liquid)
-Lose electrons easily
-Malleable
-Ductile
Where are nonmetals found on the periodic table?
Right of staircase + hydrogen
What are properties of nonmetals?
-Gases/Brittle solids (except for Br, which is liquid at 25C)
-Dull Looking/No Luster
-Poor conductor of heat and or electricity
-Gains/Shares electrons
What is the table trend for atomic radius?
Top Right-High, Bottom Left-Low
What is the table trend for Electronegativity?
Top Right-High, Bottom Left-Low
What is the table trend for ionization energy?
Top Right-High, Bottom Left-Low
What is the table trend for electron affinity?
Top Right-High, Bottom Left-Low
Which elements are diatomic when found in nature?
-Nitrogen
-Oxygen
-Fluorine
-Bromine
-Iodine
-Hydrogen