Chapter 11: Introduction to Organic Chemistry: Hydrocarbons
11.1: Organic Compounds
- Organic Chemistry: The study of carbon compounds.
- Organic Compounds: Always contain carbon and hydrogen, and sometimes other nonmetals such as oxygen, sulfur, nitrogen, phosphorus, or a halogen.
- Hydrocarbon: These organic compounds consist of only carbon and hydrogen.
- A hydrocarbon is referred to as a saturated hydrocarbon when all the bonds in the molecule are single bonds.
11.2: Alkanes
Alkanes: These are a type of hydrocarbon in which the carbon atoms are connected only by single bonds
Names of alkanes end in –ane.
- Methane, Ethane, Propane, Butane, etc.
IUPAC system: A system for naming organic compounds devised by the International Union of Pure and Applied Chemistry.
Alkanes with five or more carbon atoms in a chain are named using Greek prefixes:
- pent, hex, hept, oct, non, and dec.
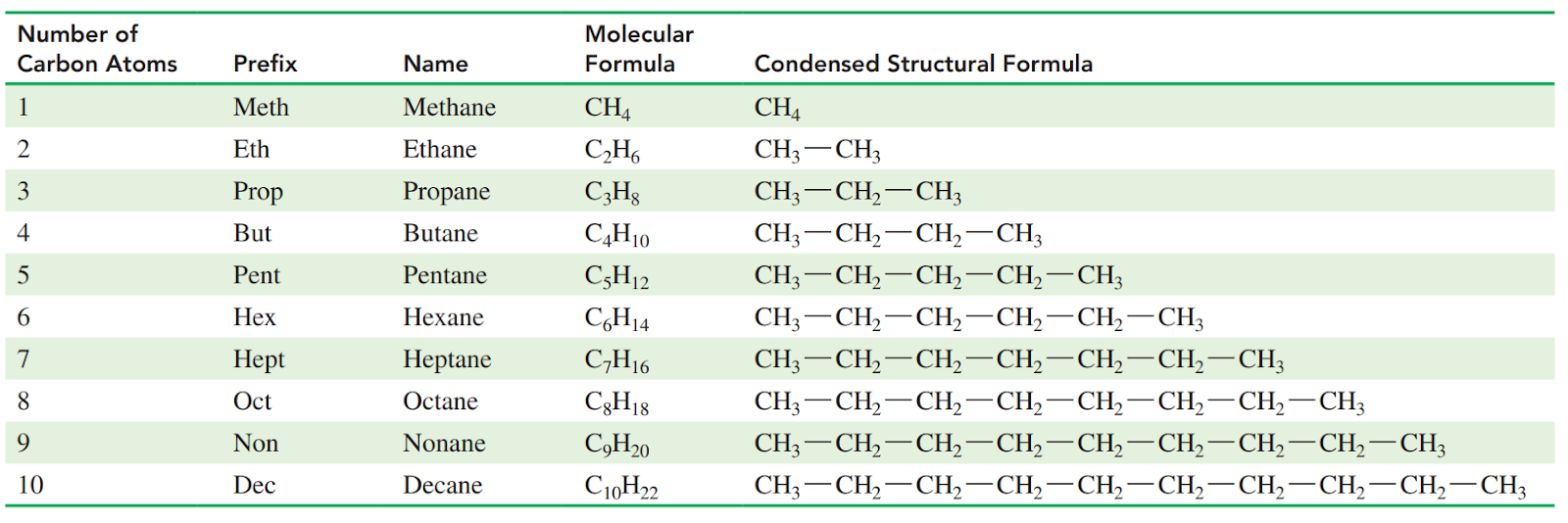
Condensed Structural Formulas
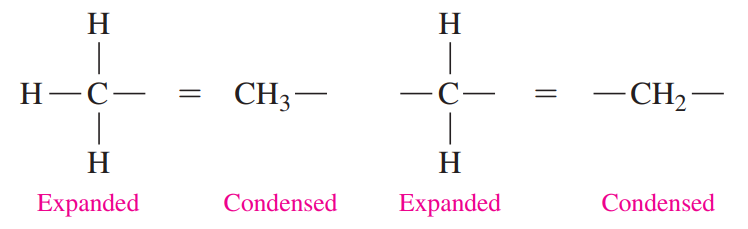
Each carbon atom and its attached hydrogen atoms are written as a group. A subscript indicates the number of hydrogen atoms bonded to each carbon atom.
Molecular formula: It gives the total number of carbon and hydrogen atoms, but does not indicate their arrangement in the molecule.
Skeletal Formula: A simplified structure that shows the carbon skeleton in which carbon atoms are represented at the end of each line or as corners.
Conformations of Alkanes
- Because an alkane has only single carbon–carbon bonds, the groups attached to each C are not in fixed positions.
- They can rotate freely about the bond connecting the carbon atoms. This motion is analogous to the independent rotation of the wheels of a toy car
- Conformations: The different arrangements that occur during the rotation of a single bond.
- Cycloalkanes: Hydrocarbons can also form cyclic or ring structures, which have two fewer hydrogen atoms than the corresponding alkanes.
- Cyclopropane: The simplest cycloalkane that has a ring of three carbon atoms bonded to six hydrogen atoms.
11.3: Alkanes with Substituents
- Substituent
- When an alkane has four or more carbon atoms, the atoms can be arranged here, which is attached to the carbon chain.
- Branched Alkane: An alkane with at least one branch.
- Structural Isomers: When the two compounds have the same molecular formula but different arrangements of atoms.
- Substituents in Alkanes
- Alkyl Group: An alkane that is missing one hydrogen atom.
- The alkyl group is named by replacing the –ane ending of the corresponding alkane name with –yl.
- When a halogen atom is attached to a carbon chain, it is named as a halo group: fluoro, chloro, bromo, or iodo.
- Haloalkanes: Halogen atoms replace hydrogen atoms in an alkane.
11.4: Properties of Alkanes
- The first four alkanes — methane, ethane, propane, and butane — are gases at room temperature and are widely used as heating fuels.
- Alkanes having five to eight carbon atoms are liquids at room temperature; they are highly volatile.
- Liquid alkanes with 9 to 17 carbon atoms have higher boiling points and are found in kerosene, diesel, and jet fuels.
- Motor oil: A mixture of high-molecular-weight liquid hydrocarbons and is used to lubricate the internal components of engines.
- Mineral oil: A mixture of liquid hydrocarbons and is used as a laxative and a lubricant.
- Alkanes with 18 or more carbon atoms are waxy solids at room temperature.
- Paraffins: They are used in waxy coatings added to fruits and vegetables to retain moisture, inhibit mold, and enhance appearance.
- Petroleum: A semisolid mixture of hydrocarbons with more than 25 carbon atoms used in ointments and cosmetics and as a lubricant.
- Alkanes are nonpolar, which makes them insoluble in water.
- However, they are soluble in nonpolar solvents such as other alkanes.
- The carbon–carbon single bonds in alkanes are difficult to break, which makes them the least reactive family of organic compounds.
11.5: Alkenes and Alkynes
- Alkenes and alkynes are families of hydrocarbons that contain double and triple bonds.
- They are unsaturated hydrocarbons because they do not contain the maximum number of hydrogen atoms, as do alkanes.
- They react with hydrogen gas to increase the number of hydrogen atoms to become alkanes, which are saturated hydrocarbons.
- Alkenes: These contain one or more carbon–carbon double bonds that form when adjacent carbon atoms share two pairs of valence electrons.
- Alkyne: A triple bond forms when two carbon atoms share three pairs of valence electrons.
- The IUPAC names for alkenes and alkynes are similar to those of alkanes.
- Using the alkane name with the same number of carbon atoms, the –ane ending is replaced with –ene for an alkene and –yne for an alkyne.
- Cycloalkenes: These are some alkenes that have a double bond within a ring structure.
11.6: Cis–Trans Isomers
- Cis–Trans Isomers: These are compounds that have different configurations because of the presence of a rigid structure in their molecule.
- Cis Isomer: An isomer of an alkene in which similar groups in the double bond are on the same side.
- Trans Isomer: An isomer of an alkene in which similar groups in the double bond are on opposite sides.
11.7: Additional Reactions
- The most characteristic reaction of alkenes is the addition of atoms or groups of atoms to the carbon atoms in a double bond.
- It occurs because double bonds are easily broken, providing electrons to form new single bonds.
- Hydrogenation
- H atoms add to each of the carbon atoms in a double bond of an alkene.
- The double bonds are converted to single bonds in alkanes.
- A catalyst is used to speed up the reaction.
- Hydration
- An alkene reacts with water (H — OH).
- A hydrogen atom (H —) from water forms a bond with one carbon atom in the double bond, and the oxygen atom in —OH forms a bond with the other carbon.
- The reaction is catalyzed by a strong acid such as H2SO4.
11.8: Aromatic Compounds
- In 1825, Michael Faraday isolated a hydrocarbon called benzene, which had the molecular formula C6H6.
- A molecule of benzene consists of a ring of six carbon atoms with one hydrogen atom attached to each carbon.
- Aromatic Compounds: Family of benzene compounds.
- In 1865, August Kekulé proposed that the carbon atoms in benzene were arranged in a flat ring with alternating single and double bonds between the carbon atoms.