MED CHEM QUIZ 8 💊
1/129
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
130 Terms
Resting Platelets
Act as vascular sentries monitoring endothelial integrity
In absence of injury → circulate freely without interaction
Endothelium prevents contact with vessel wall substances that cause activation
Injury exposes subendothelial collagen → platelets adhere & activate
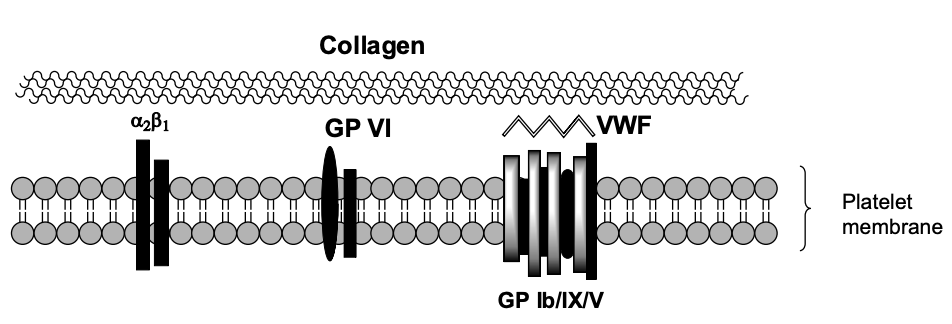
Step 1: Platelet Contact with Damaged Endothelium
Unactivated platelet contacts exposed collagen
Mediated by:
Gp Ib/IX/V complex binding to von Willebrand factor (vWF)
vWF tethers platelet to collagen
Additional receptors: Gp VI and Integrin α2β1 (Gp Ia/IIa)
Step 2: Platelet Activation
Binding to collagen → activation → morphological change (spiky pseudopods)
Activated platelets secrete granular contents via exocytosis:
ADP
Thromboxane A₂ (TxA₂)
Serotonin
Platelet-activating factor
Thrombin
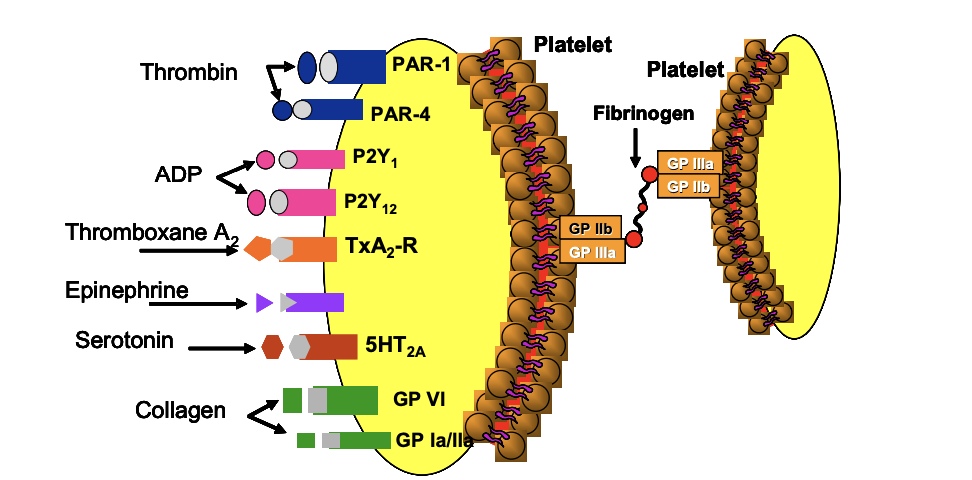
Step 2: Propagation of Platelet Activation
Released mediators (ADP, TxA₂, etc.) bind to receptors on nearby platelets
→ ↑ intracellular Ca²⁺, ↓ cAMP
→ activates more plateletsFinal Common Pathway: Activation of Gp IIb/IIIa receptors, allowing fibrinogen binding
Step 3: Platelet Aggregation
Fibrinogen binds to Gp IIb/IIIa receptors on multiple platelets → cross-linking → aggregation
Creates initial hemostatic plug → trapped by fibrin
Amplification loop: each activated platelet activates more
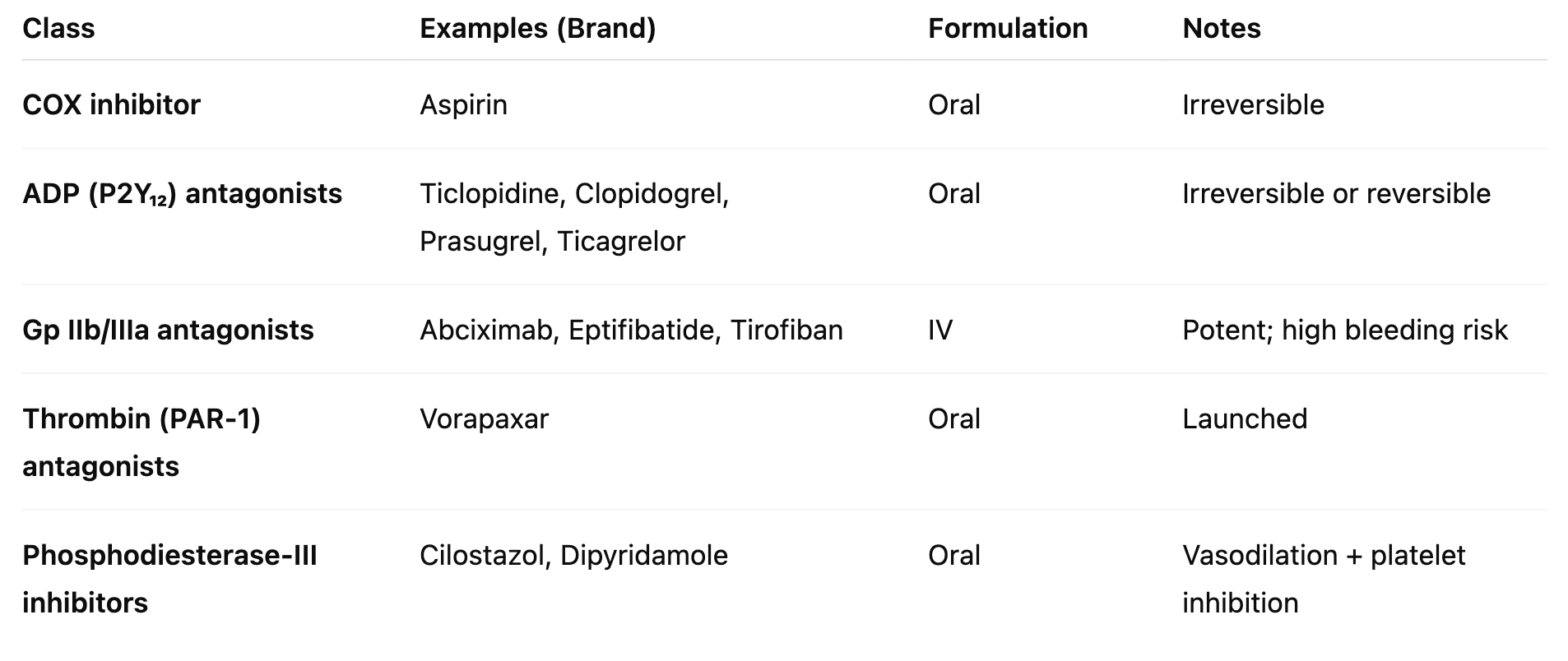
Major Classes of Antiplatelet Agents
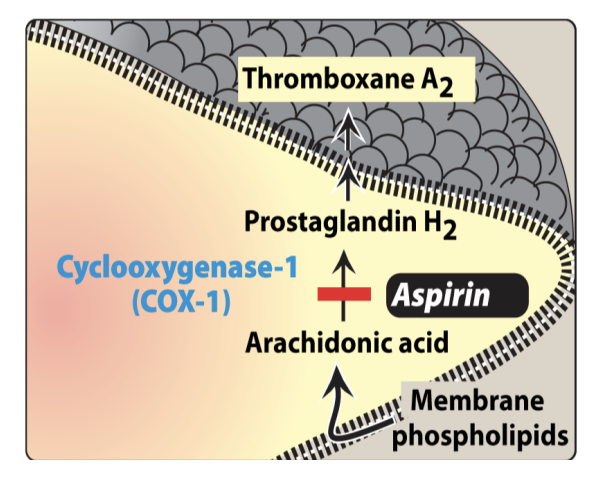
COX-1 Inhibitors
Inhibit TxA₂ Biosynthesis
Platelet activation → phospholipase activation → releases arachidonic acid (AA)
COX-1 converts AA → PGH₂, → TxA₂
TxA₂ promotes more platelet activation
COX-1 inhibitors (e.g. Aspirin) → inhibit TxA₂ → stop activation
COX-1 = constitutive; COX-2 = inducible
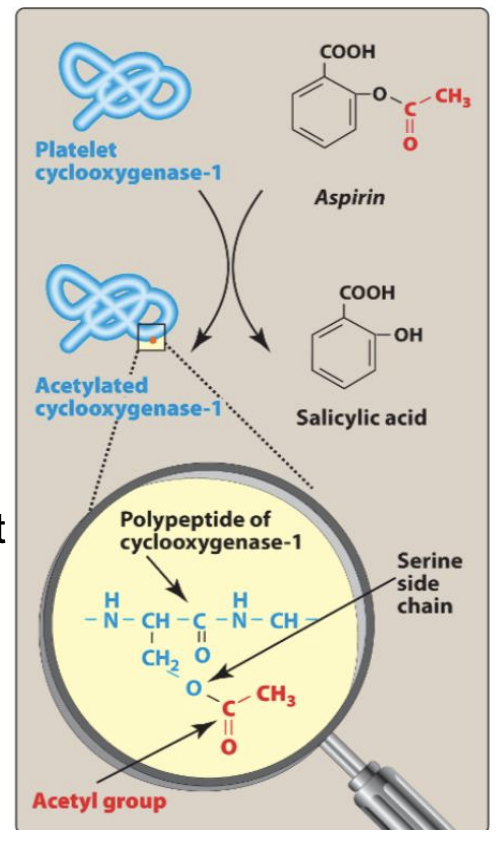
Aspirin MOA
MoA: Irreversible COX-1 inhibitor → ↓ TxA₂ synthesis and acetylates Ser529 on COX-1.
Platelets can’t resynthesize COX-1 → effect lasts 10 days
Unique: Only NSAID with antithrombotic efficacy (others reversible)are
____ is the only NSAID that has antithrombotic efficacy
Aspirin
Aspirin
MOA: Irreversible COX-Enzyme inhibitor by acetylating the serine on the COX-enzyme.
Indications:
Acute coronary syndrome (ACS) → ↓ CV mortality by 23%
Secondary prevention post-MI/stroke
Primary prevention: Risk vs benefit (bleeding risk)
AEs:
GI bleeding
↑ bleeding with other antithrombotics
Aspirin resistance: failure to inhibit TxA₂ → ↑ CV risk

ADP (P2Y₁₂) Receptors
P2X₁: ATP-activated → Ca²⁺ mobilization
P2Y₁: ADP-activated → shape change (low [ADP])
P2Y₁₂: ADP-activated → sustained aggregation (high [ADP])
How does low concentrations of ADP bring about platelet shape change, whereas higher concentration aggregation?
At low concentration P2Y1 is activated (EC50 = 0.3 uM) →shape change.
At higher concentration P2Y12 is activated (EC50 = 2 uM) → aggregation.
P2Y₁₂ Antagonists (ADP Antagonists)
Irreversible: Ticlopidine, Clopidogrel, Prasugrel
Reversible: Cangrelor, Ticagrelor (-grelor)
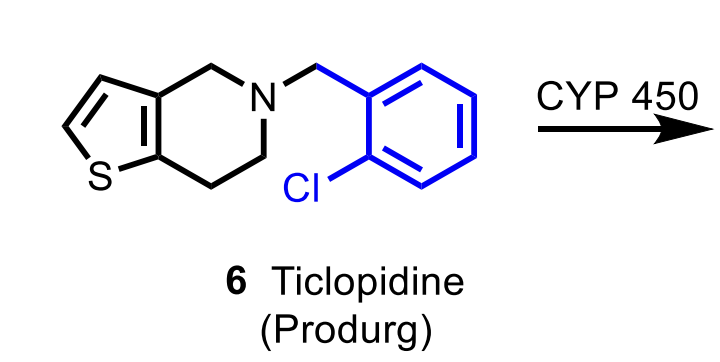
Ticlopidine
MoA: Irreversible P2Y₁₂ antagonist (prodrug → CYP activation → covalent disulfide bond)
Use:
Prevent stroke/TIA
Adjunct with aspirin after stent placement
AEs:
TTP, aplastic anemia, neutropenia, thrombocytopenia, bleeding
Notes: Replaced by clopidogrel
Ticlopidine
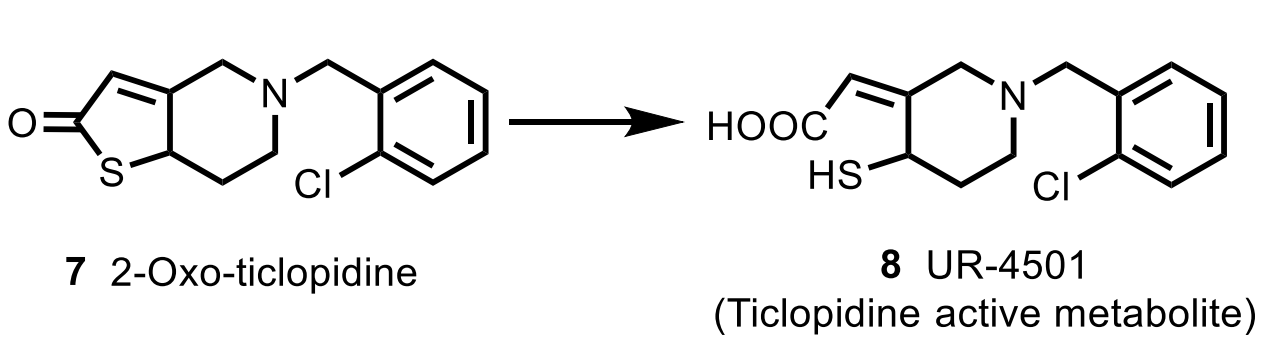
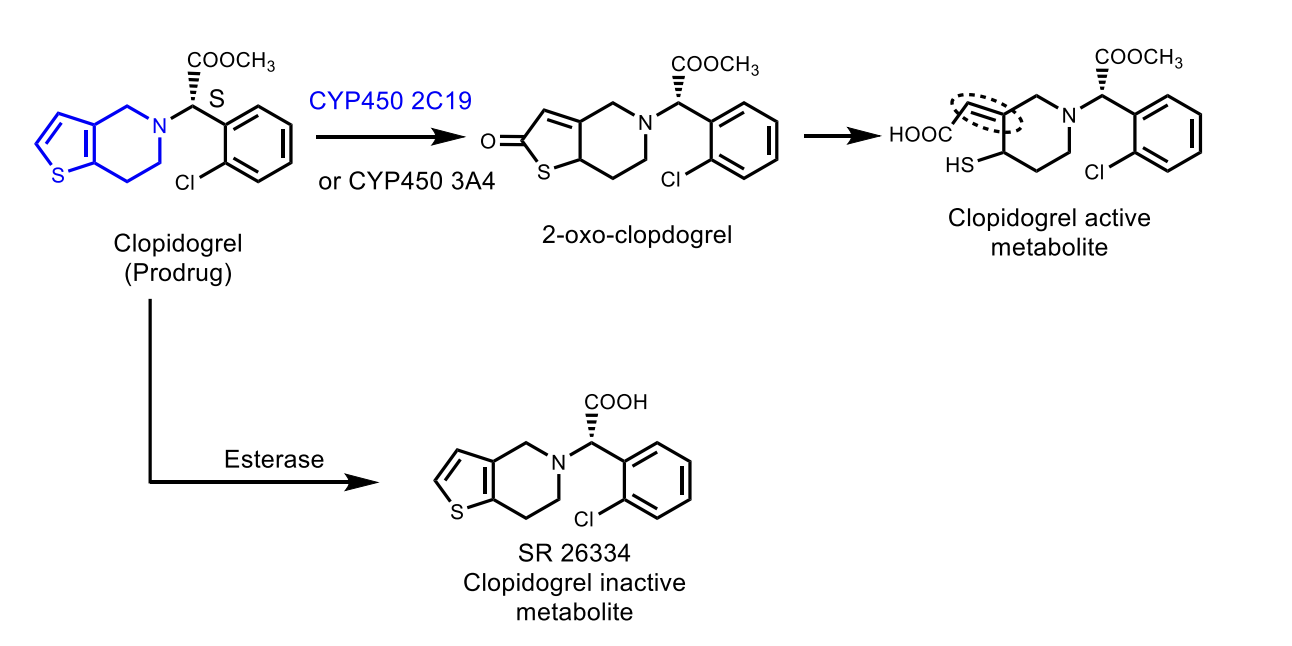
Clopidogrel
MoA: Irreversible P2Y₁₂ antagonist (prodrug → CYP activation → disulfide bond)
Use:
Secondary prevention after MI, stroke, PAD
ACS prophylaxis with aspirin
PCI (with or without stent)
AEs: Bleeding, rare TTP
Resistance: CYP2C19/CYP3A4 polymorphism → ↓ activation
Preferred over ticlopidine (safer, better data)
Clopidogrel
Activation of Clopidogrel
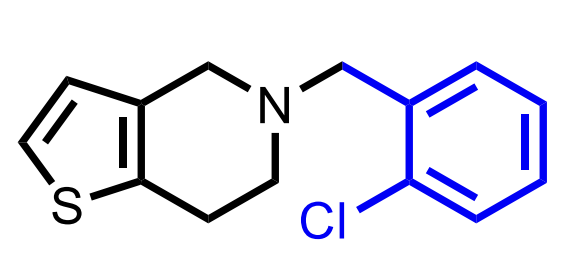
CC: Thienopyridines
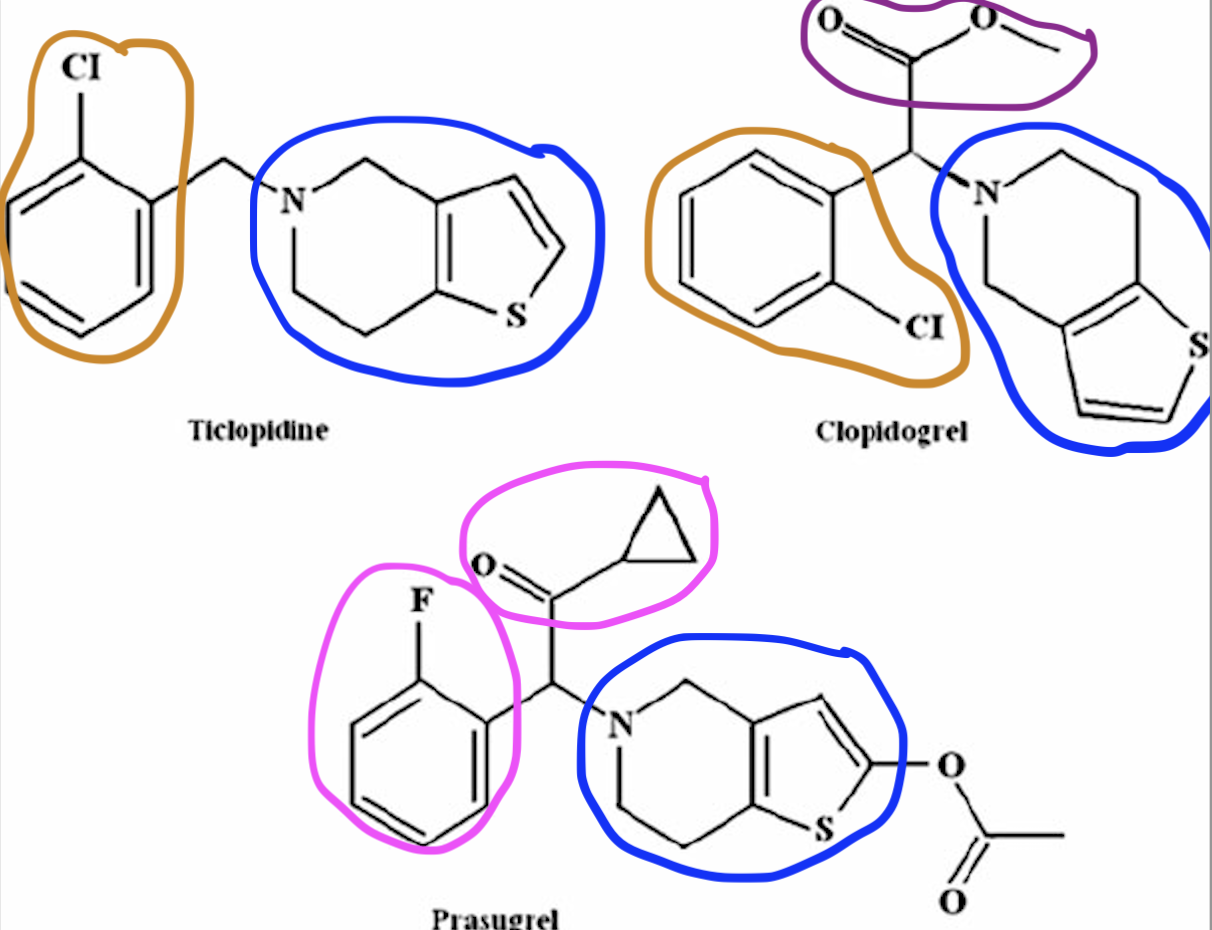
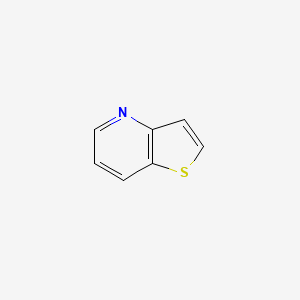
Common Structural Motif for the thienopyridines (Clopidogrel, Ticaglerol).
Prasugrel
MoA: Irreversible P2Y₁₂ antagonist (prodrug; activated by esterase → stable metabolite)
Use: ACS (with aspirin)
Better PK and stronger effect than clopidogrel
AEs: Major bleeding (⚠ BBW)
Contraindicated: history of TIA/stroke or age >75
-Grelor
Reversible P2Y₁₂ antagonist
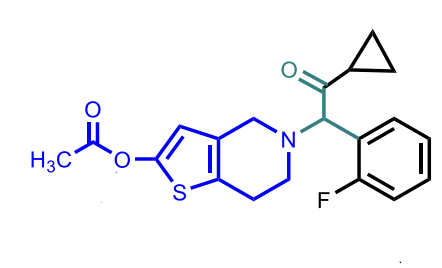
Prasugrel
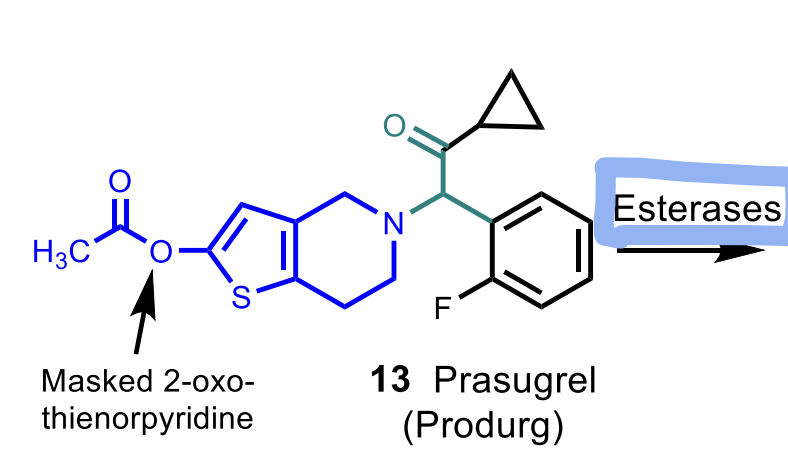
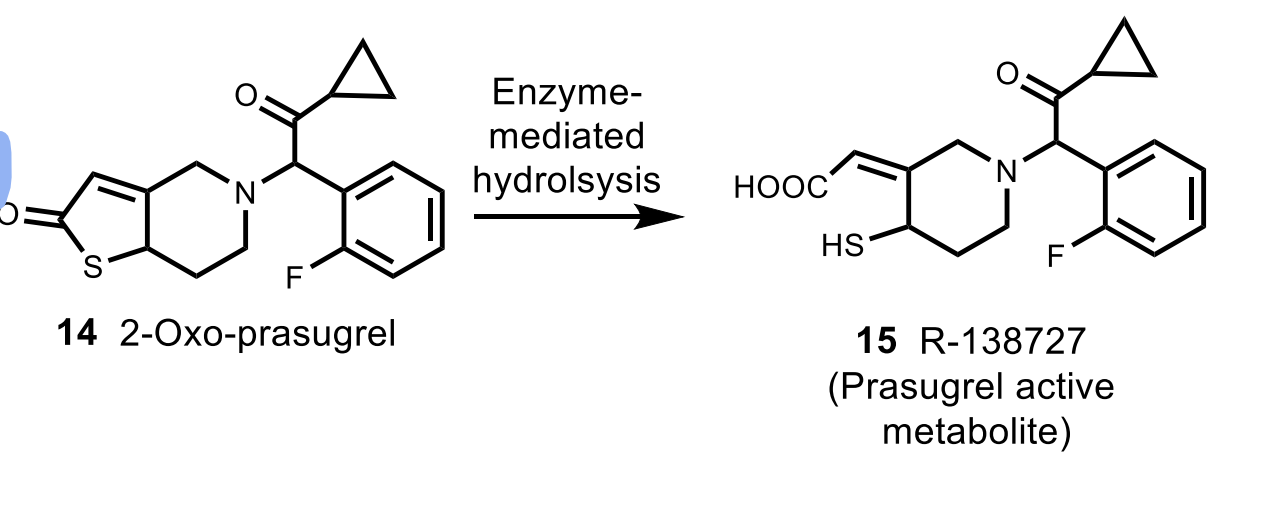
Metabolism is by esterases (**differs from the other thienopyridines)
All irreversible thienopyridines are ___
prodrugs and is activated by CYPs (exception: Prasugrel which is activated by esterases).
Cangrelor
Brand Name: Kengreal®
MoA: Reversible P2Y₁₂ antagonist (IV only)
Use: During PCI to reduce periprocedural MI
AEs: Bleeding

Cangrelor
Ticagrelor
Brand Name: Brilinta
MoA: Reversible allosteric P2Y₁₂ antagonist
Does NOT require metabolic activation
Use: Secondary prevention in ACS
AEs: Dyspnea, bleeding
Aspirin restriction: High-dose aspirin (>100 mg) ↓ effectiveness
Active metabolite: 30–40% plasma concentration of parent drug

Ticagrelor
Gp IIb/IIIa Antagonists
MoA: Block final common pathway of platelet aggregation (fibrinogen binding)
Very potent but ↑ bleeding risk
Drugs:
Abciximab – monoclonal antibody
Eptifibatide – cyclic peptide
Tirofiban – small-molecule non-peptide
All IV formulations
Abciximab
Type: Chimeric monoclonal antibody (human + murine Fab)
MoA: Binds Gp IIb/IIIa → blocks fibrinogen & vWF binding
Use:
Adjunct in PCI (with heparin/aspirin)
Unstable angina, MI prophylaxis
t½: 72 hours (effects 24–48 h post-infusion)
AEs: Major bleeding
Expensive due to antibody production

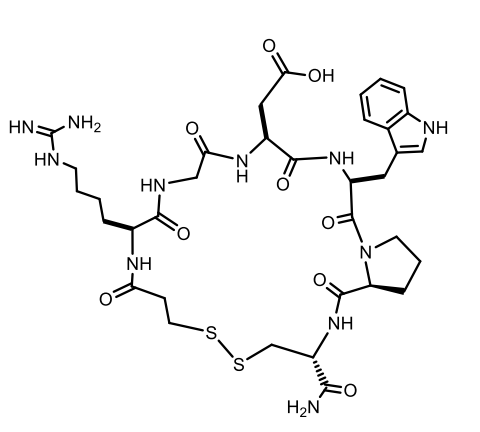
Eptifibatide
Eptifibatide
Type: Cyclic heptapeptide (snake venom analog)
MoA: Reversible inhibition of fibrinogen/vWF binding to Gp IIb/IIIa
Use: ACS, PCI
Formulation: IV
t½: ~2.5 hours
AEs: Bleeding
Tirofiban
Type: Low MW non-peptide reversible Gp IIb/IIIa antagonist
Formulation: IV
t½: 2 hours
Use: ACS – reduces death, MI, ischemia
AEs: Bleeding
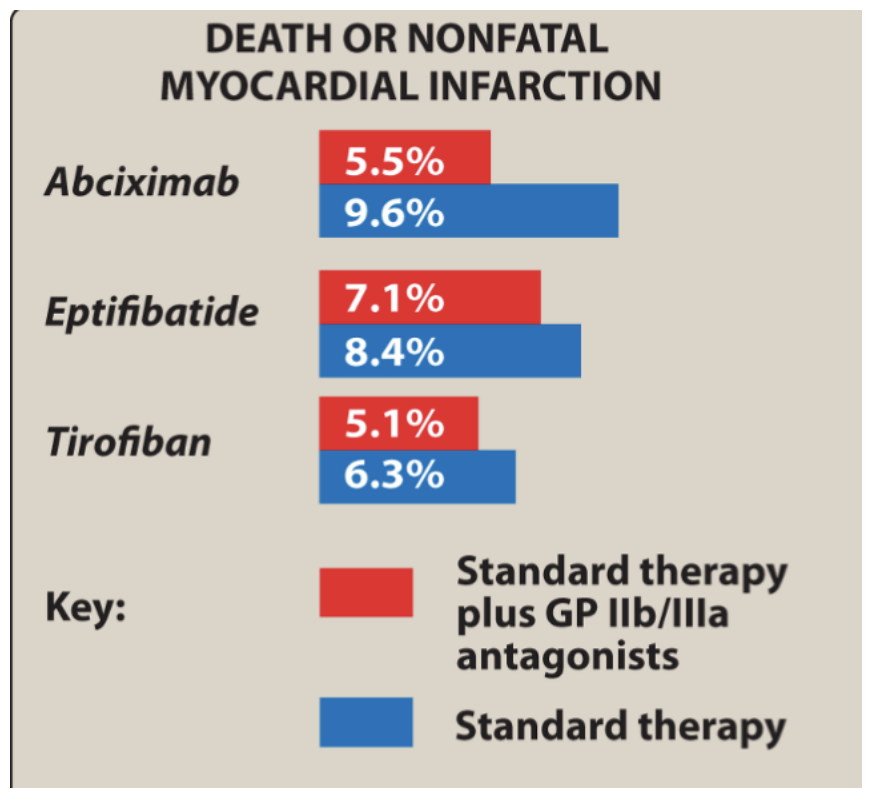
Outcome results of GPIIb/IIIa antagonists
Thrombotic Thrombocytopenic Purpura (TPP) is a major ADR of which thienopyridine?
Ticlopidine
Vorapaxar
MOA: PAR-1 Antagonist that blocks platelet activation by inhibiting thrombin’s PAR-1 receptor.
Brand Name: Zontivity®
Does NOT inhibit thrombin’s ability to convert fibrinogen → fibrin (JUST INHIBITING PLATELET AGGERATION)
Works by preventing the “tethered ligand” from activating PAR-1
Derived from structural studies of natural product himbacine
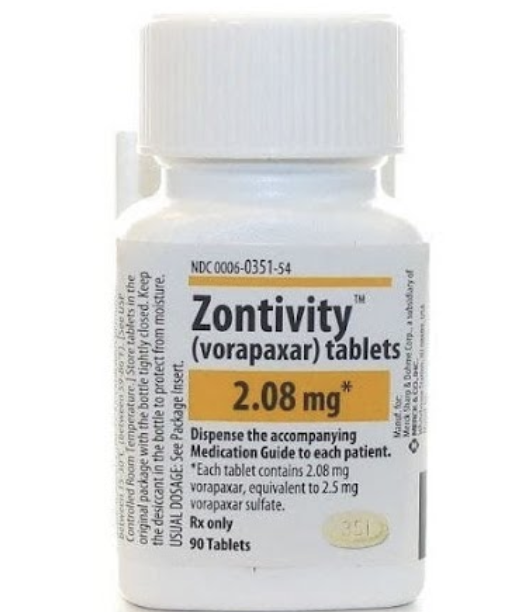
MOA of Vorapaxar
Vorapaxar
Vorapaxar was discovered from what?
Natural product Himbacine which is a muscarinic antagonist.
Himbacine has no PAR-1 activity on it’s one
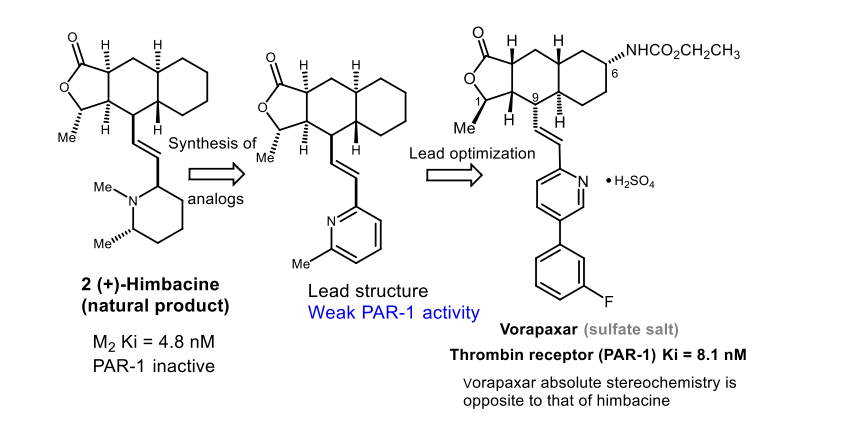
Himbacine → Muscarinic Antagonist that lead to Vorapaxar → Stereochemistry is opposite to that Vorapaxar
Vorapaxar Indications
dded to aspirin + ADP antagonist for secondary prevention in:
Prior MI
Peripheral arterial disease (PAD)
Contraindicated in history of stroke or intracranial bleeding
Given orally
Vorapaxar Pharmacokinetics
Oral bioavailability ≈ 100%
Long effective half-life: 3–4 days
Metabolism: CYP3A4, CYP2J2
Active metabolite: M20 (~20% exposure)
Inactive metabolite: free amine
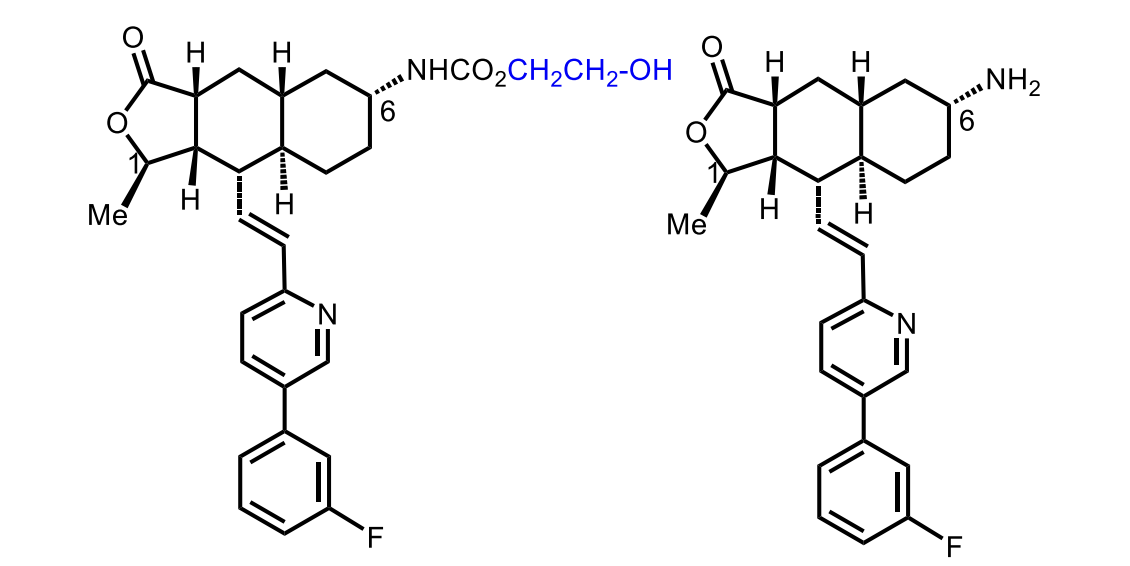
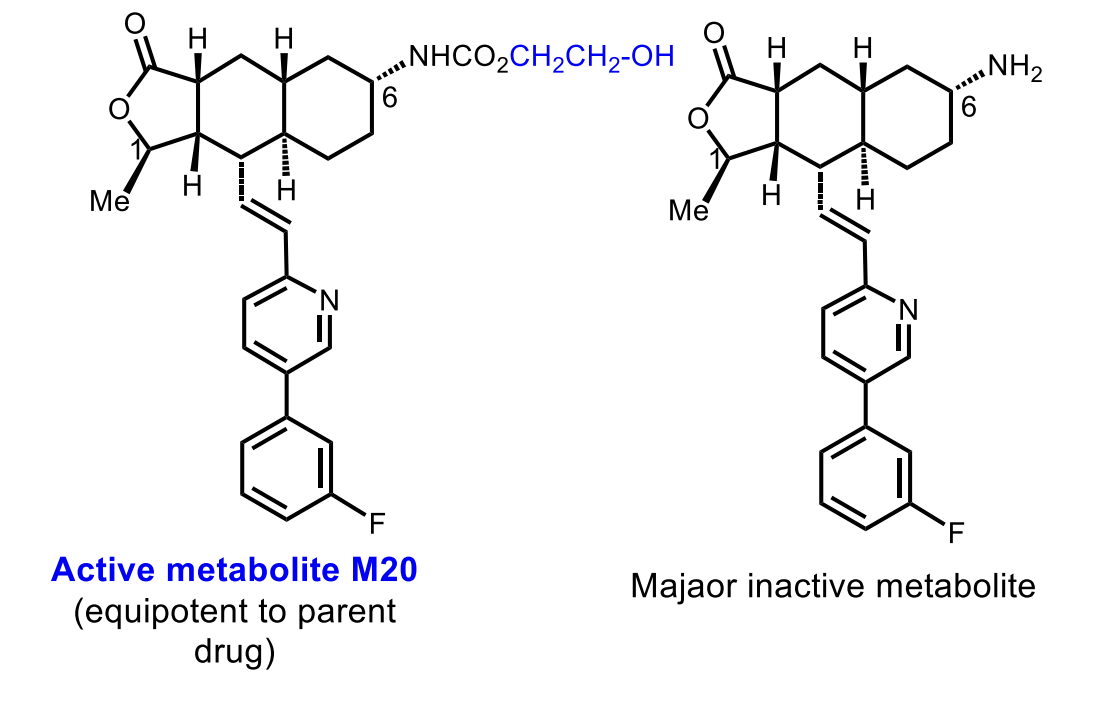
Of Vorapaxar
The major metabolite is inactive!
Cilostazol MoA
PDE III inhibition → ↑ cAMP in platelets & vessels
Platelets: inhibits aggregation
Vascular tissue: vasodilation
Blocks adenosine uptake → decreased aggegration
Improves lipid profile (↓ triglycerides, ↑ HDL)
Cilostazol

Cilostazol Uses
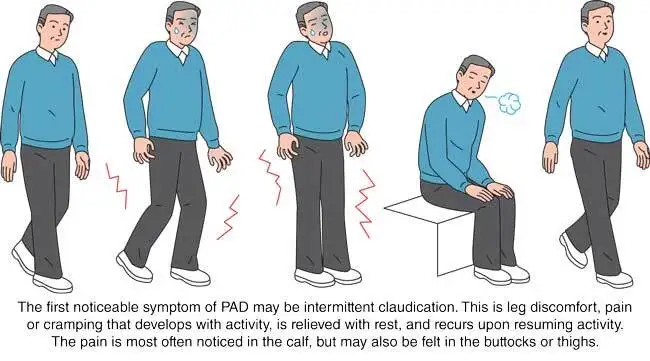
FDA: Intermittent claudication in PAD
Non-FDA: Buerger’s disease, diabetic vascular sclerosis, chronic cerebral ischemia
Cilostazol PK / AEs / Warnings
Oral; food ↑ absorption
Two active metabolites
Contraindicated in heart failure
Use caution with other PDE-3 inhibitors or cardiac disease
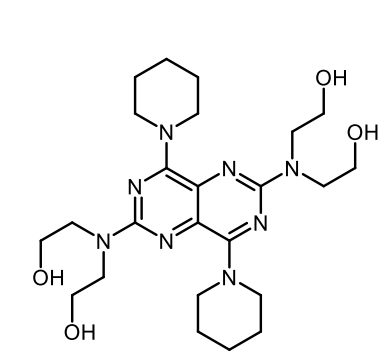
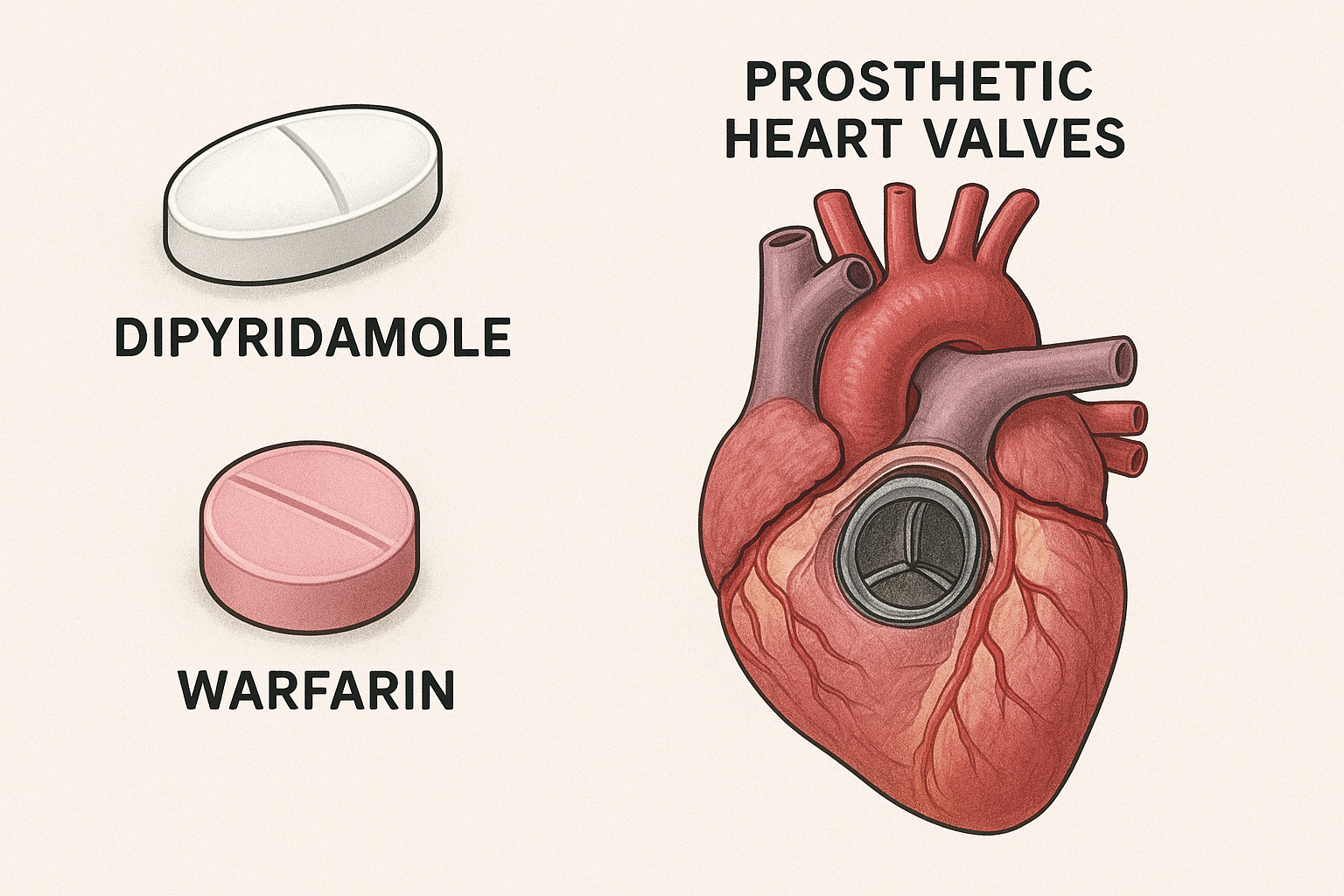
Dipyridamole
Dipyridamole MOA
Dual mechanism (same as Cilostazol):
PDE-III inhibition → ↑ cAMP → ↓ platelet aggregation
Blocks adenosine reuptake
Also PDE-V inhibition → peripheral vasodilation
Dipyridamole Use
Commonly combined with aspirin
Combined with warfarin to prevent emboli from prosthetic heart valves (ONLY FDA indication)
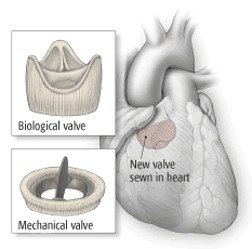
Dipyridamole + Warfarin
Prevent emboli from prosthetic heart valves (ONLY FDA indication)
Aggrenox
Dipyridamole + aspirin, used to prevent stroke after TIA/stroke

Dipyridamole AE
Avoid as monotherapy in elderly (GI issues, fainting/orthostasis)
Fibrinolytic Drug Purpose
Dissolve fibrin clots (polymerized fibrin)
Used in MI, PE, DVT, arterial thrombosis
Used when PCI unavailable
High bleeding risk (lyse normal + pathological clots)
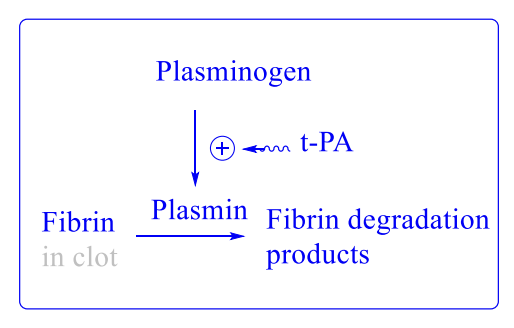
Endogenous Plasmin System
Plasminogen converted → plasmin (fibrin-digesting enzyme)
Major activator: tPA from endothelium
tPA regulated by PAI-1 and PAI-2
Thrombolytic Drugs MOA
Act like t-PAs and convert plasminogen to plasmin to dissolve clots.
Suffix: “teplase”
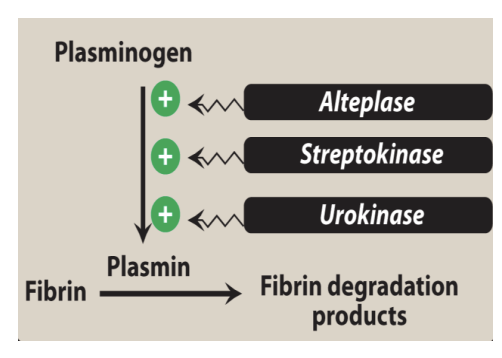
Types of Thrombolytic Drugs
First Generation: Streptokinase
Second Generation: Alteplase
Third Generation: Tenecteplase; Reteplase
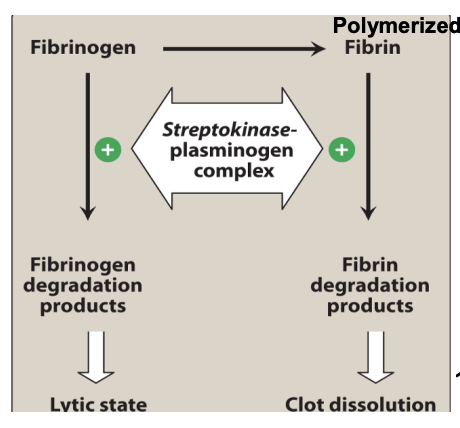
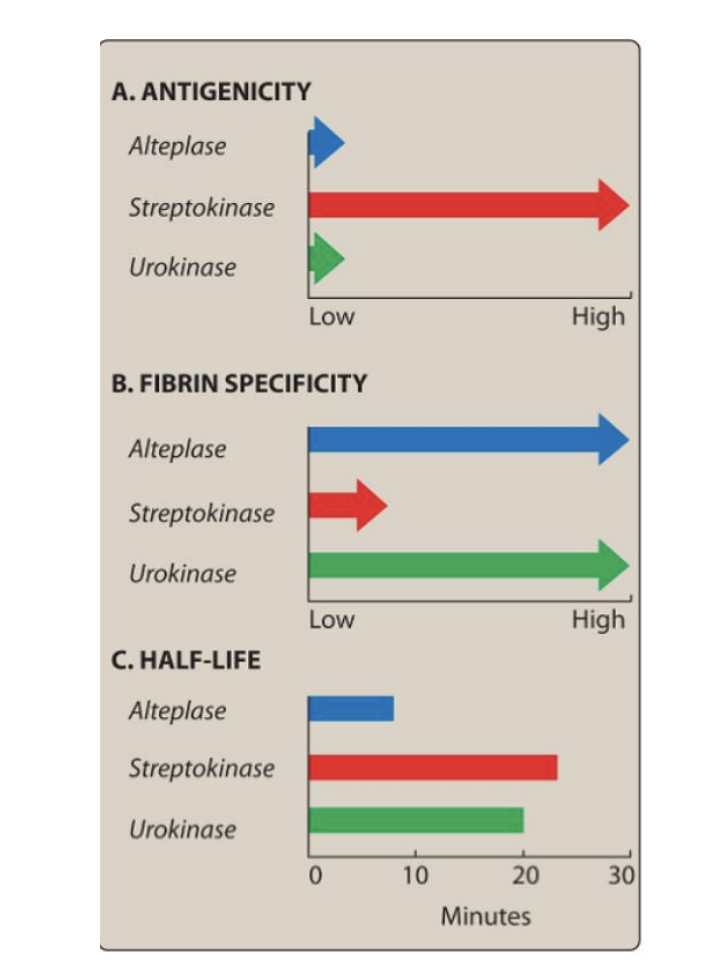
Streptokinase
Bacterial protein from β-hemolytic streptococci
Forms 1:1 complex with plasminogen → converts free plasminogen → plasmin (act like tPA)
NOT fibrin-specific → systemic fibrinolysis
Uses: PE, DVT, MI, arterial thrombosis, shunt occlusion
Cheap (cost-effective)
Streptokinase AEs
Bleeding due to non-specific lysis activity and degrrades fibrinogen (precursor to fibrin) and Factors V and VII.
Also more plasmin means that more clots are dissolved and increased bleeding risk.
Hypersensitivity (rash/fever/anaphylaxis)
Ineffective if antibodies from prior strep infection neutralize it (since it’s a foregin protein).
Antibody presence → need high dose to overcome resistance
Aminocaproic Acid
Antidote if too much bleeding occurs from streptokinase administration → promotes a thromobitic state.
Streptokinase PK
IV formulation
Very short t½ < 30 min
Anistreplase
Prodrug (acylated with anisoyl group at the active serine site plasminogen–streptokinase complex)
Longer half-life: ~90 min
More clot-specific than streptokinase
Given as rapid IV bolus (3–5 min)
Better reperfusion due to longer action
Urokinase
Human enzyme (not antigenic) → like Streptokinase
Directly converts plasminogen → plasmin
Also directly degrades fibrin & fibrinogen (*ADDITIONAL EFFECT THEN OTHER KINASES*).
t½ ~15 min; cleared by liver
Use: PE, coronary artery thrombosis (off-label MI, DVT)
AE: Bleeding (most common)
“from UR body" → not antigenic”
Alteplase
MOA: Second-generation recombinant t-PA
Fibrin-selective at low doses (this can lead to hemorrhage)
Activates plasminogen bound to fibrin
Short t½ < 5 min
Use: MI, massive PE, acute ischemic stroke (within 3 hours)
AE: GI & intracranial bleeding
Reteplase
Brand Name: Retavase
Deletion mutant of tPA (lacks first 172 aa)
↓ fibrin specificity but longer half-life: 14–18 min
Use: Acute MI
Off-label: catheter occlusions, DVT
CC: First vs Second Generation Thrombolytic Agents
Tenecteplase
Brand Name: TNKase®
Modified tPA with 3 point mutations and inhibits PAI-1 (plasminogen inhibitor)
↑ t½ (17 min)
15× higher fibrin specificity vs alteplase
Use: Acute MI (give ASAP)
Off-label: ischemic stroke
Tranexamic Acid
Used as an antidote for too much bleeding/hemorrhage caused by fibrinolytic therapy
Tranexamic Acid
Blocks lysine sites on plasminogen → prevents conversion to plasmin
Reverses fibrinolytic bleeding
Oral/IV; renal elimination; t½ ≈ 2 h
Uses: heavy menses, dental bleeding on anticoagulants, fibrinolytic reversal
AE: thrombosis, hypotension, muscle necrosis
ε-Aminocaproic Acid
Same MoA as tranexamic acid
Oral/IV, renal elimination, t½ ≈ 2 h
Use: active bleeding due to fibrinolysis, postop/control bleeding
AE: thrombosis, hypotension, muscle necrosis

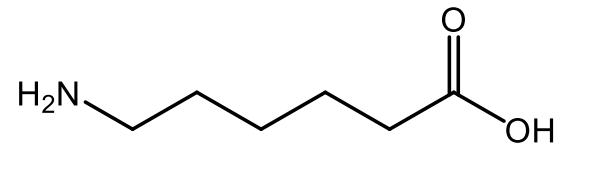
ε-Aminocaproic Acid
What are the 3 major steps involved in platelet aggregation?
Platelet adhesion, platelet activation, and platelet aggregation via GpIIb/IIIa cross-linking.
What is the mechanism of propagation of platelet activation?
Activated platelets release mediators (ADP, TxA₂, serotonin, thrombin, PAF) that bind receptors on nearby resting platelets, increasing Ca²⁺ and decreasing cAMP → activating more platelets.
What are the major biochemical mediators involved in the propagation of platelet activation?
ADP, thromboxane A₂ (TxA₂), serotonin, thrombin, and platelet-activating factor (PAF).
What is the final convergent pathway in platelet activation (that leads to platelet aggregation)?
Prothrombin → Thrombin
Activation of GpIIb/IIIa receptors, allowing fibrinogen to cross-link platelets.
What is the antiplatelet mechanism of action of aspirin?
Irreversible inhibition of COX-1 → decreased thromboxane A₂ formation → reduced platelet activation.
What is unique about the mode of binding (and inactivation) of aspirin on COX enzymes?
Aspirin covalently acetylates Ser529 on COX-1, making the inhibition irreversible for the platelet’s lifespan.
What are the 2 major antiplatelet indications for aspirin?
Acute coronary syndrome (ACS) and secondary prevention after MI or stroke.
What is known about the relative risk of patients with aspirin resistance?
Aspirin-resistant patients have higher cardiovascular risk.
What are the 3 major purinergic receptors and what are their main activators?
P2X1: Activated by ATP
P2Y1: Activated by ADP → platelet shape change
P2Y12: Activated by ADP → sustained aggregation
Which ADP receptors cause platelet shape change? Which cause aggregation?
Shape change: P2Y1
Aggregation: P2Y12
Which are the irreversible P2Y12 antagonists? Which are the reversible ones?
Irreversible: Ticlopidine, clopidogrel, prasugrel
Reversible: Cangrelor, ticagrelor
What is the molecular mechanism of irreversible P2Y12 antagonism?
Formation of a covalent disulfide bond with receptor cysteine residues.
What are the two major AEs of ticlopidine?
TTP and hematologic toxicity (neutropenia, aplastic anemia).
What are the two major indications of ticlopidine?
Prevention of TIA/stroke and adjunct therapy with aspirin after stent placement.
Know the prodrug nature of ADP antagonists such as ticlopidine.
Ticlopidine, clopidogrel, and prasugrel must be metabolically activated into their active thiol metabolites.
Is the major circulating metabolite of clopidogrel active?
No — the major carboxylic acid metabolite is inactive.
What chemical bond causes irreversible inactivation of ADP receptors?
A disulfide covalent bond to receptor cysteines.
What are the 3 major indications for clopidogrel?
Secondary prevention after MI/stroke/PAD, ACS with aspirin, and PCI (with or without stent).
Are any ADP antagonists indicated for primary prevention?
No
What is the reason for clopidogrel resistance?
CYP2C19 and CYP3A4 genetic polymorphisms or drug interactions reducing activation.
How is prasugrel activation different from ticlopidine/clopidogrel? Is its active metabolite stable?
Prasugrel uses esterase activation (not CYP), and its active metabolite is stable.
What is the use restriction for prasugrel?
Must be used with aspirin.
What is the major indication for prasugrel?
Treatment of ACS.
What are the 2 boxed warnings for prasugrel?
Major bleeding risk and contraindicated in prior TIA/stroke or age >75.
What is the half-life of cangrelor? What is it used for?
t½ ≈ 2.6 minutes; used during PCI to prevent periprocedural MI.
Is ticagrelor reversible or irreversible? Is it a prodrug?
Reversible; not a prodrug.
Is ticagrelor a competitive or allosteric P2Y12 binder?
Allosteric binder — binds a different site from ADP.