Chemistry Exam Review (Unit 1 - 4)
1/74
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
75 Terms
What is Empirical and Theoretical Knowledge
Empirical knowledge = knowledge that comes from investigation and observation
Theoretical knowledge = knowledge that explains scientific observations
What is IUPAC
IUPAC = The International union of pure and applied chemistry
What is SATP
standard ambient temperature and pressure (SATP) is also used in chemistry as a reference standard. It is defined as the following: Temperature: 25 degrees Celsius (298.15 degrees Kelvin) Pressure: 1 atm (101.325 kPa)
What is the Atomic Number, Atomic(Mass) Number and Atomic mass Units
Atomic number = z (the unique number of protons in one atom of an element)
Mass number = a (number of protons and neutrons an element has)
Atomic mass units = 1/12 the mass of carbon-12
Describe Alkali Metals (Group 1)
Elements: Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Cesium (Cs), Francium (Fr).
Key Points:
Highly reactive, especially with water.
Form strong bases (alkaline solutions) when combined with water.
Typically form +1 cations.
Soft metals, low density.
Describe Alkaline Earth Metals (Group 2)
Elements: Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba), Radium (Ra).
Key Points:
Less reactive than alkali metals but still reactive.
Form oxides that are basic (alkaline).
Typically form +2 cations.
Important for biological processes (e.g., calcium in bones).
Describe Transition Metals (Groups 3-12)
Elements: Iron (Fe), Copper (Cu), Nickel (Ni), Zinc (Zn), Silver (Ag), Gold (Au), etc.
Key Points:
Exhibit multiple oxidation states.
Form colored compounds.
Often act as catalysts in chemical reactions.
High melting points and densities.
Conduct electricity and heat well.
Describe Halogens (Group 17)
Elements: Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I), Astatine (At).
Key Points:
Highly reactive nonmetals.
Form salts when combined with metals.
Typically form -1 anions.
Exist in different states at room temperature (F₂ and Cl₂ are gases, Br₂ is a liquid, I₂ is a solid).
Describe Noble Gases (Group 18)
Elements: Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), Radon (Rn).
Key Points:
Inert due to a full valence shell of electrons.
Used in lighting (neon lights) and refrigeration (helium).
Low reactivity makes them stable and nonflammable.
What is the Octet Rule and a full octet
Full or stable octet = an electron arrangement where the valence shell is filled with 8 valence electrons (2 for hydrogen and helium)
Octet rule = a generalization stating that when atoms combine, they tend to achieve 8 valence electron
What is an Ion, cation, Anion, Valence Shell and Multivalent Valence
Ion = a charged entity formed when an atom gains or loses one or more electron
Cation = positively charged ions formed by the removal of one or more electron for the valence shell of a neutral atom
Anion = a negatively charged ion formed by the addition of one or more electrons to a neutral atom
Valance = the charge of an ion; the combining capacity of an atom determined by the number of electron that it will lose, add, or share when it reacts with other atoms
Multivalent = the property of having more than one possible valence
What are Isotopes
Isotopes = a form of an element in which the atoms have the same number of protons as all other forms of the element, but a different number of neutrons
Isotopic abundance = the percentage of given isotope in a sample of element
Mass spectrometer = a measuring instrument used to determine the mass and abundance of isotopes
What are Metalloids, Groups, Periods and Period Law
Metalloid = an element that has properties of both metals and nonmetals
Groups = a column of elements in the periodic table; sometimes referred to as a family
Period = a row in the periodic table
Period law = a rule, developed from many observations, stating that when the elements are arranged in order of increasing atomic number, their peropetes show a periodic recurrence of gradual change
What is the Atomic Radius and Effective Nuclear Charge
Atomic radius (a measurement of the size of an atom, usually expressed in hectometers (pm); the distance from the center of an atom to the outermost electrons
ENC = effect nuclear charge is the net force expressed by an electron in an atom due to the positively charged nucleus
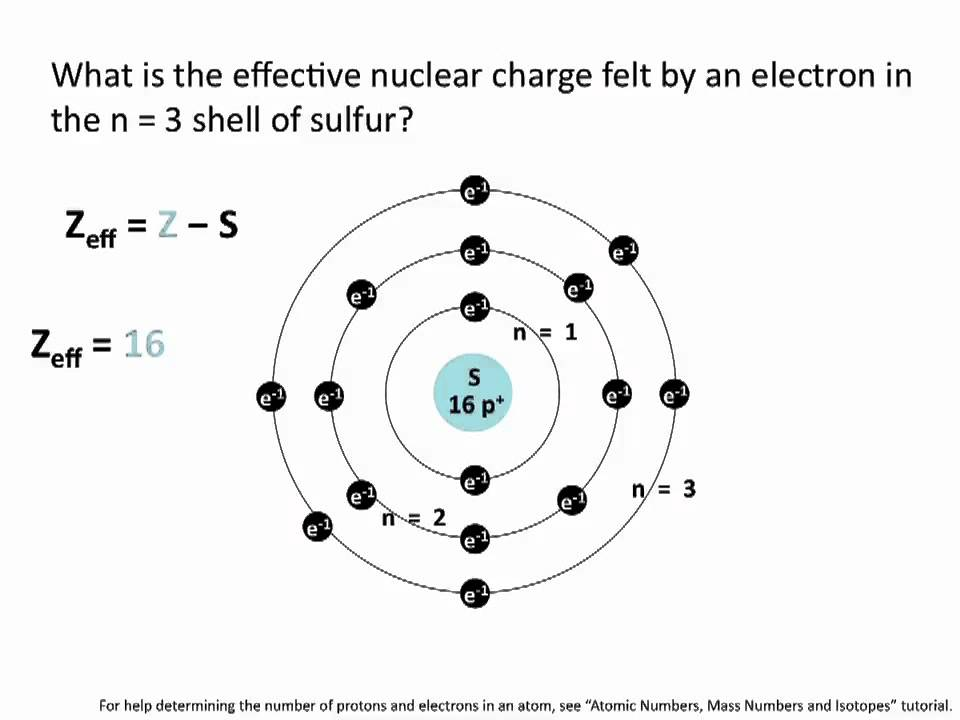
What is Ionic Radius, Ionization energy and Electron affinity
Ionic radius = a measurement of the size of an ion, usually expressed in picometers (pm); the distance from the center of an ion to the outermost electron
Ionization energy = the quantity of energy required to remove an electron from an atom or ion in the gaseous state
Electron affinity = the energy change that occurs when an electron is added to a neutral atom in the gaseous state
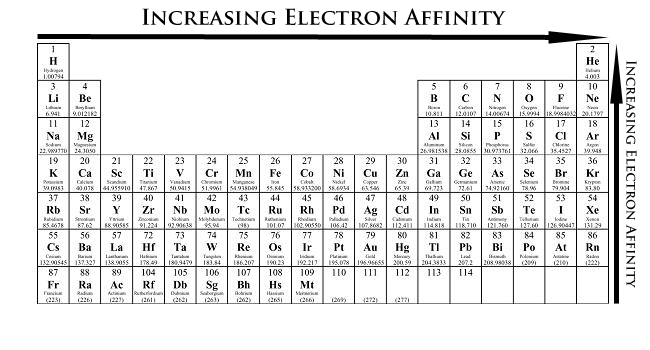
What is the Periodic Trend regarding Electron Affinity
E.A. decreases down a group:
Each additional period represents a new shell.
ENC stays nearly constant.
Force of attraction between an e- and the nucleus decreases as an e- gets farther away.
Extra e- is not easily welcomed as the size increases.
E.A. increases from left to right across a period:
Valence shell number is the same.
Number of outer e- and p+ increases.
Number of core e- stays the same, so ENC increases.
Force of attraction between an e- and the nucleus increases as an e- gets closer.
Extra e- is more easily welcomed as the size decreases.
Electrons can be added in succession:
Known as the first E.A., second E.A., etc.
Successive additions may be more difficult since the e- will be added to a negative ion (anion).
Elements of Group 18:
Tend not to gain e-.
Have stable electron arrangements
What are the 3 types of Bonds
Ionic Bond
attraction between a cation (+) and an anion (-)
Covalent Bond
attraction between two neutral atoms as a result of the sharing of an e- pair between the two atoms (the e- pair attracts both nuclei and so holds both atoms together)
Metallic Bond
attraction between metal atoms - not on exam
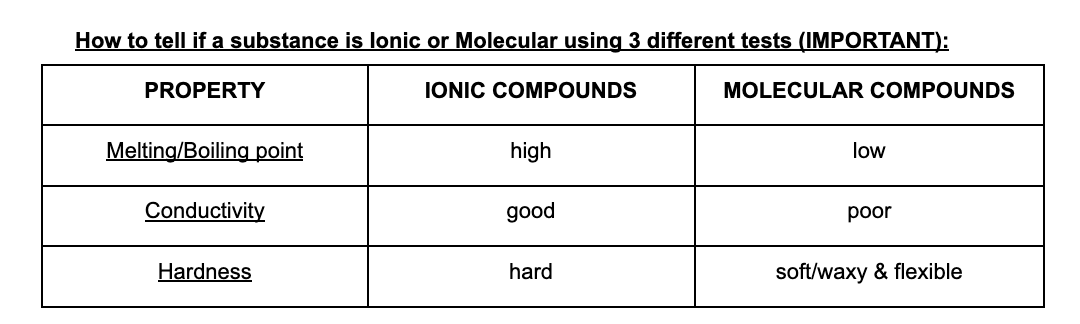
What are the Properties of Ionic compounds and Ionic Bonding
Properties of Ionic compounds
Hard and brittle/high melting and boiling points/conduct electricity as molten liquid but not as acid/conduct electricity when dissolved in water
Ionic Bonding = the electrostatic force of attraction between a positive ion and negative onion; a type of chemical bong
What are Molecular elements, Molecular compounds and the properties of Molecular compounds
Molecular element = a pure substance composed of molecules made up of two or more atoms of the same element
Molecular compound = a pure substance composed of molecules made up of two or more non-metallic elements
Properties of Molecular Compounds
Lower Melting and Boiling Points: Weaker intermolecular forces lead to generally lower melting and boiling points compared to ionic compounds.
Poor Electrical Conductivity: Typically do not conduct electricity in solid or dissolved states because they do not form ions.
Varied Physical States: Can be solid, liquid, or gas at room temperature based on the strength of intermolecular forces.
Solubility: Often soluble in non-polar solvents; solubility in water depends on molecular polarity.
Molecular and Ionic comparison
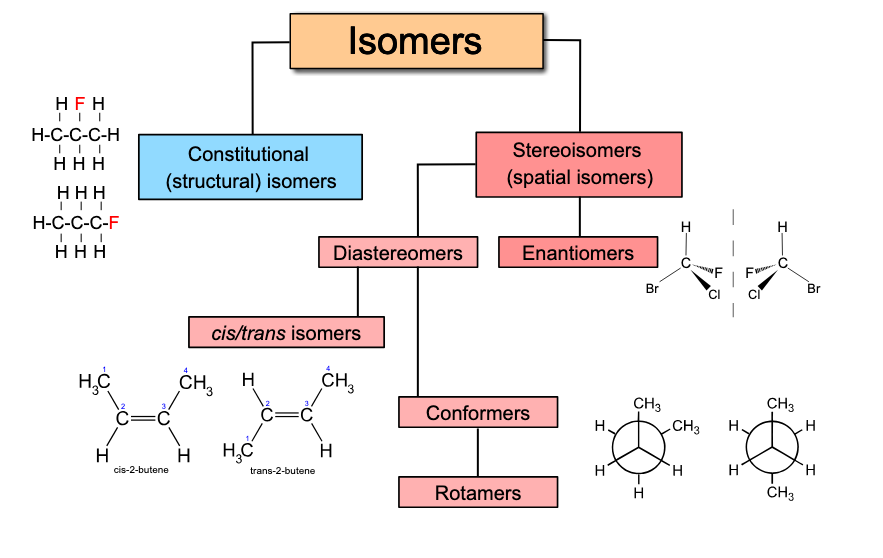
What are Structural Isomers
Basically means: Same chemical formula but different connectivity of atoms
Example: Butane and isobutane
What are Covalent Bonds, Non-polar Covalent bonds and Polar covalent bonds
Covalent Bond
The bond that results from the sharing of a pair of electrons by two atoms
Polar covalent bonds
0.4 or less - non-polar
0.5 - 1.7 - polar covalent
1.7 - 4 Ionic
Polar covalent bond = a covalent bond formed between atoms with significant difference in NE resulting in a bond with a localized positive and negative charge/pole.
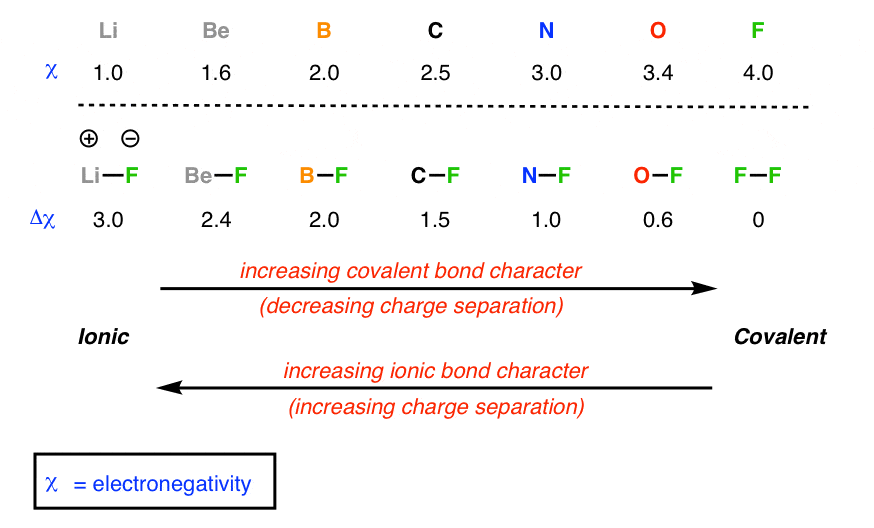
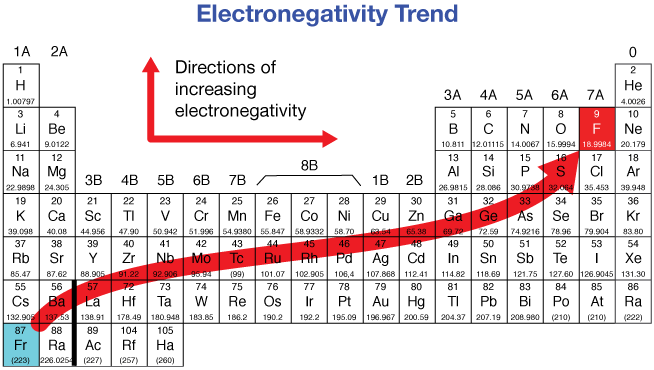
What is Electronegativity
Electronegativity = the ability of an atom to attract bonding electron to itself
What are Binary Compounds?
Binary compounds = a compound that consists of ions of only two elements
Examples
Na3P - Sodium phosphide
MgO - Magnesium oxide
K2S - potassium sulfide
AlN - Aluminum nitrate
Al2O3 - Aluminum oxide
These Binary compounds can also be (Nonmetal + Nonmetal)
what are Multivalent Metals + Nonmetals
a compound consisting of one Multivalent Metal (Charges of Copper. +1, +2. Iron. +2, +3., Mercury. +1, +2 ect. mixed with a Non-mental compound (Oxygen etc)
What are Hydrates?
Hydrates are compounds that can include water in its lattice crystalline structure
-
What are Acids
There are 2 types of Acids (Binary acids, Oxyacids)
Binary acids are (Hydrogen Mixed + Non-mental without oxygen) - come with prefix (Hydro)
Oxyacids are (Hydrogen + Non-metal with Oxygen) -
Prefix
If the polyatomic ion ends in "-ate," the suffix of the acid is changed to "-ic acid."
If the polyatomic ion ends in "-ite," the suffix of the acid is changed to "-ous acid."
What are the 3 Neutralization Reactions
Acid + Hydroxide Base Neutralization:
Will create/produce water and salt (ionic compound).Acid + Carbonate Base Neutralization:
Will create/produce water, carbon dioxide, and salt (ionic compound.Acid + Bicarbonate Base Neutralization:
Will create/produce water, carbon dioxide, and salt (ionic compound.
Naming Flowcart
What is Bond Polarity
When two atoms are joined by a polar covalent bond, the negative pole, where the electron density is greater, is indicated by “8-.” The positive pole, where the electron density is less, is indicated by “8+.”
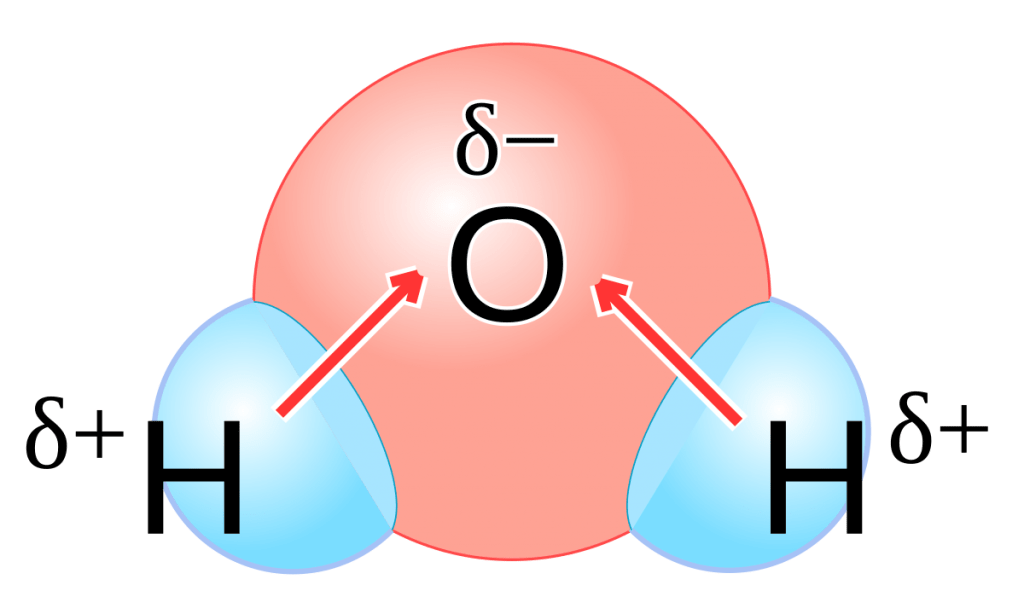
What are Polar and Non-polar molecules
Polar molecular = a molecular in which uneven distribution of electrons results in a positive charge at one end and a negative charge at the other end
Non-polar molecule = a molecule in which the electrons are equally distributed among the atoms, resulting in no localized charges
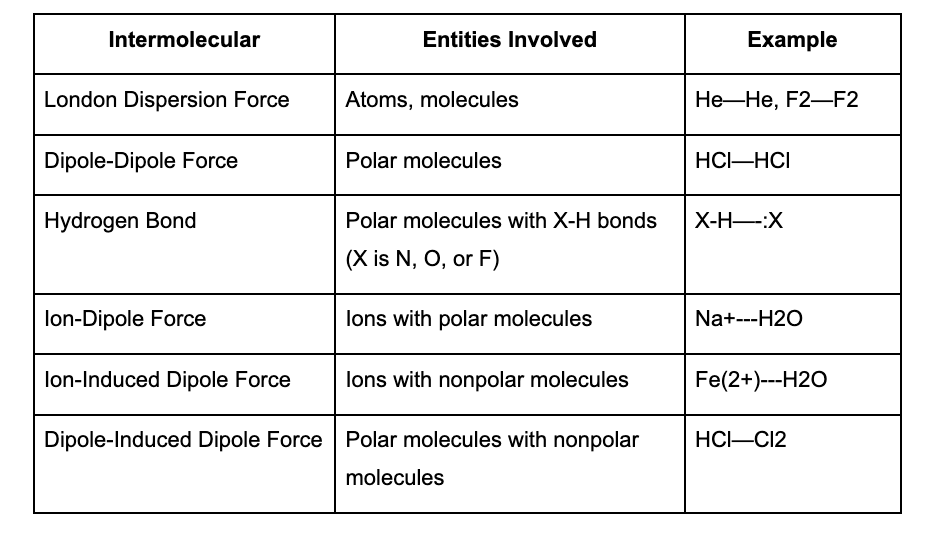
What are Intermolecular and Intramolecular forces
Intermolecular Forces:
Definition: Forces of attraction or repulsion between molecules.
Types:
London Dispersion Forces: Weakest, present in all molecules, stronger in larger/heavier atoms.
Dipole-Dipole Interactions: Occur between molecules with permanent dipoles (polar molecules).
Hydrogen Bonds: Strong type of dipole-dipole interaction, occurring when H is bonded to N, O, or F.
Ion-Dipole Forces: Occur between an ion and a polar molecule.
Intramolecular Forces:
Definition: Forces that hold atoms together within a molecule.
Types:
Covalent Bonds: Sharing of electron pairs between atoms.
Ionic Bonds: Transfer of electrons from one atom to another, resulting in attraction between oppositely charged ions.
Metallic Bonds: Delocalized sharing of free electrons among a lattice of metal atoms.
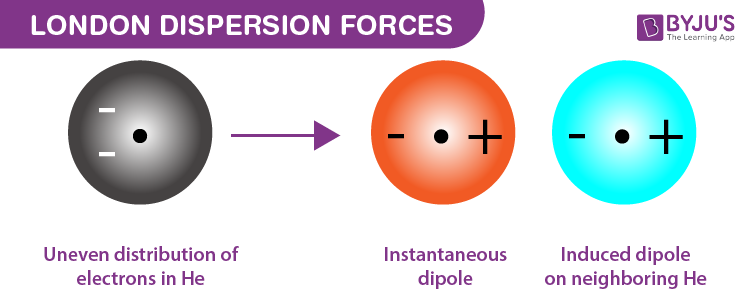
What is the London Dispersion Force
A weak attractive force acting between all entities, including non-polar molecules and unbonded atoms, caused by the temporary imbalance of electrons within entities
These are temporary short-lived forces that form because of an imbalance of electrons.
Whenever a dipole is created by another molecule or atom, it's called an induced dipole (it doesn't last very long, so its a temporary induced dipole)
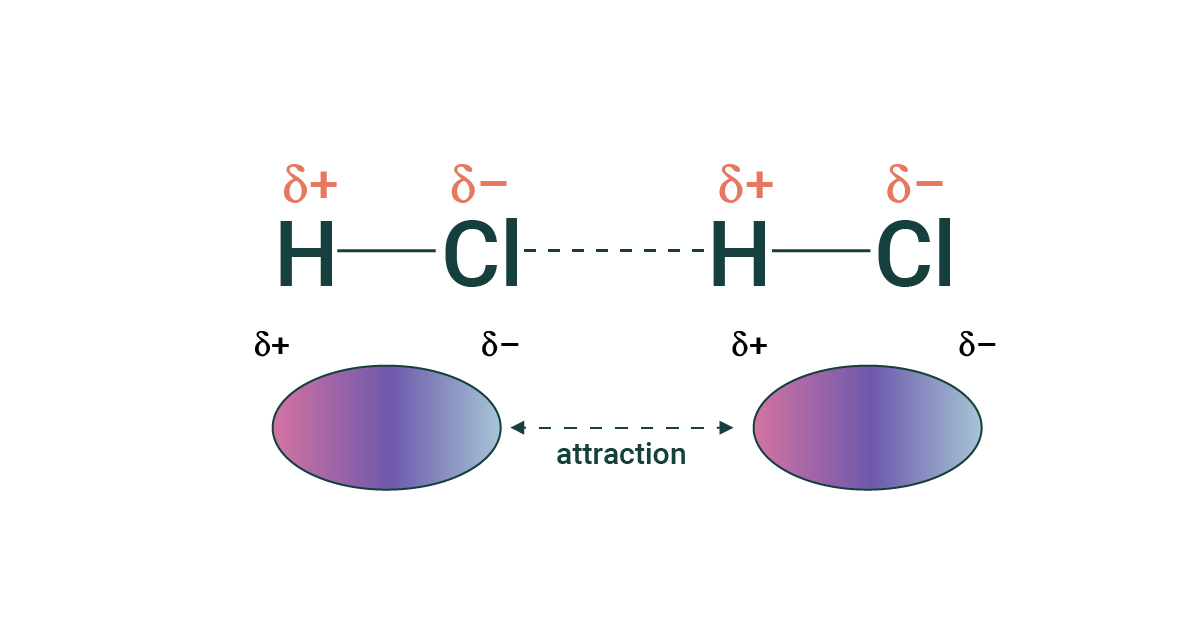
What is the Dipole-Dipole force?
The intermolecular forces between oppositely charged ends of polar molecules is called a dipole-dipole force. In general, these forces are relatively strong (intermolecular forces). They occur between all polar molecules.
Essentially oppositely charged ends of neighboring dipoles will ATTRACT while like charged ends of neighboring dipoles will REPEL. This network will act to hold the entities in a sample together.
What are Hydrogen Bonds
An unusually strong dipole-dipole force between a hydrogen atom attached to a highly electronegative atom (N, O or F) and a highly electronegative atom in another molecule
Molecules that have X-H covalent bonds can donate and accept H-bonds, but molecules that have an X atom not covalently bound to H can only accept H-bonds
X = variable for other elements
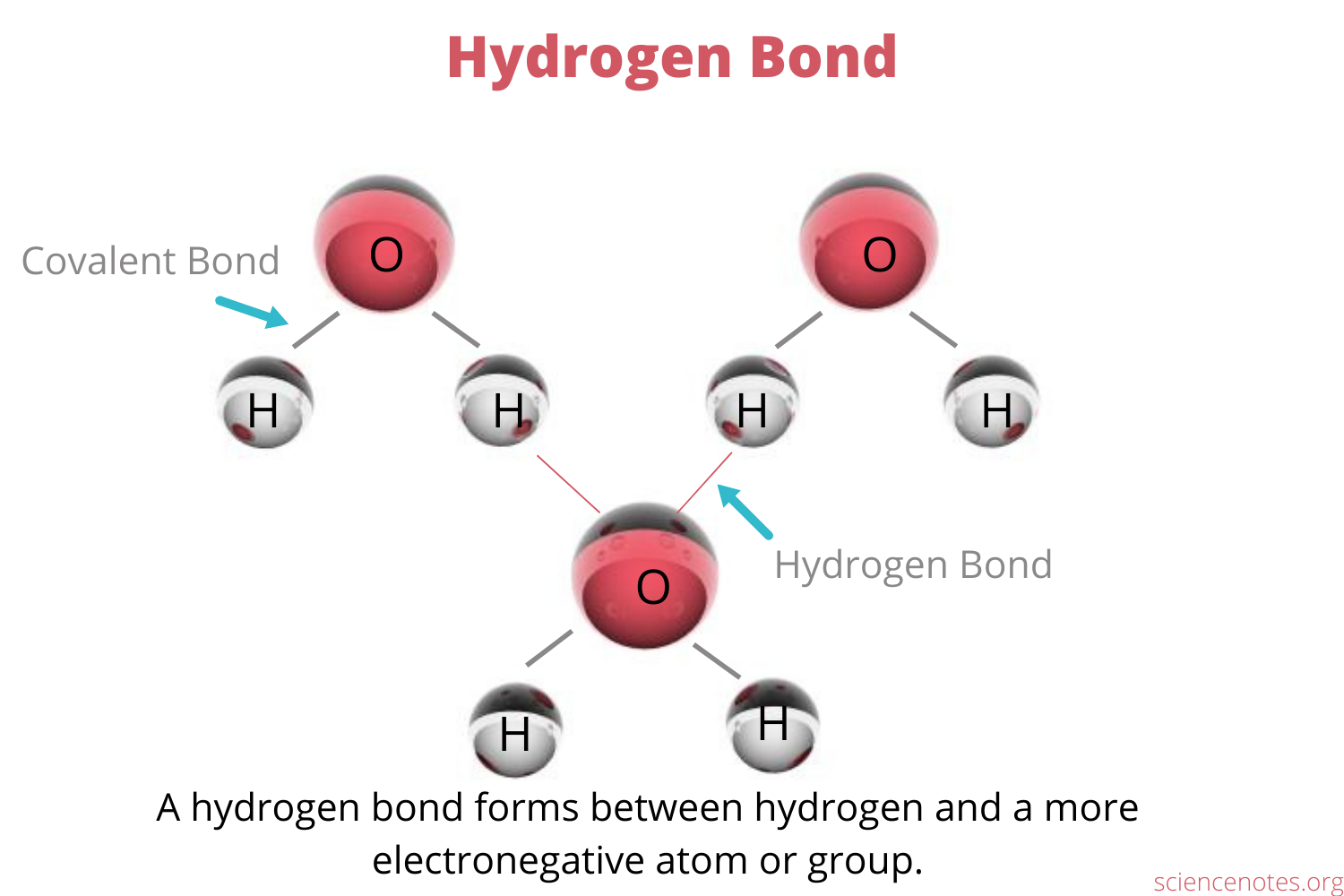
Table Comparing Intermolecular Forces
Explain the Covalent bonds of Carbons Ions in Diamond
Diamond consists of carbon atoms each bonded to four other carbon atoms via strong covalent bonds, forming a three-dimensional lattice.
The tetrahedral bonding structure gives diamond its hardness, high melting point, and thermal conductivity.
Diamond is an excellent electrical insulator due to the absence of free electrons.
Its unique optical properties make diamond highly valued as a gemstone.
The strong covalent bonds also contribute to its chemical inertness and stability.
What is surface tension
a phenomenon, caused by forces of attraction between molecules that lead to the formation of a skin-like film on the surface of a liquid.
Surface tension → explains how small objects that are denser than water can remain on the surface of water.
What are Precipitates, Catalysts and the law of conservation of mass
Chemical Reactions: A process in which one or more substances change into one or more new substances
Peripiate: A solid produced as a result of the reactions of two solutions
Catalyst: a substance that makes a chemical reaction occur faster without itself begin consumed in the reaction
Law of conservation of mass: the statement that, during a chemical reaction, the total mass of reactant equals the total mass of products.
What is the Evidence of a chemical reactions
Evidence of a chemical reaction includes color change; absorption or release of energy; production of a gas (except evaporating or boiling of a liquid); and formation of a precipitate.
What is a Synthesis Reactions
Synthesis reaction: a reaction in which two reactants combine to make a larger or more complex product; general pattern
A + B → AB
We can apply the pattern of synthesis reactions to these situations. Since the reactants are non-metals, the products will be molecular compounds.
Synthesis reactions + Hydrogen
Hydrogen usually forms molecular compounds and molecular compounds.
What is a Decomposition Reaction
Decomposition reactions: a reaction in which a large or more complex compound breaks down to form two (or more) simpler products: general pattern AB → A + B
Decomposition reactions usually need energy to get started even for reactions that release a lot of energy.
What is a Single Displacement Reaction
Single Displacement reaction: reaction in which an element displaces another element in a compound, production an new, prodigy a new compound and a new element: general pattern: A + BC → AC + B
What is the Activity Series Chart
Activity Series: a list of elements arranged in order of their observed reactivity in single displacement reactions.
One element can displace elements below it from compounds in solution but cannot displace elements above it
The further apart two elements are, the more likely it is that the displacement reaction will occur quickly.
how precipitate reactions occur and what happens when a precipitate forms.
In precipitate reactions, two solutions containing positive ions (cations) and negative ions (anions) are mixed. The positive ions from one solution attract and bond to the negative ions from the other solution.
If the resulting compound is only slightly soluble in the liquid, a precipitate forms.
What is a Double Displacement Reaction
Double displacement reaction: a reaction in which elements in two compounds displace each other or trade places, producing two new compounds; general pattern
AB + CD → AD + CB
Double displacement reactions vary, resulting in different products. Some produce precipitates, some release gasses, and others result in solutions that are more neutral than the reactant solutions. We can classify double displacement reactions according to the type of products they generate.
Define Solution, Solute, Solvent, and Solubility
Solution = a homogeneous mixture of two or more substances.
Solute = the substance that dissolves in a solvent
Solvent = the substance that dissolves the solute
Solubility = the quantity of solute that dissolves in a given quantity of solvent at a given temperature
What is a Combustion Reactions and what are the different types
Combustion → a chemical reaction in which a fuel burns in oxygen to produce combustion products and often, a flame
Complete Combustion of a hydrocarbon
The combustion (burning) of a hydrocarbon in plentiful of a hydrocarbon in a plentiful supply of oxygen to produce carbon dioxide, water and energy.
Result = Less sooty clean and very hot flames
Blue Flame = complete combustion
1:5 ratio required (fuel to oxygen)
Incomplete combustion
The combustion (bunting) of a hydrocarbon in a limited supply of oxygen; products may include carbon dioxide, carbon monoxide, carbon soot, water and energy
Results = Sooty, Yellow and cooler flames
1:3.5 Ration required (fuel to oxygen)
How can Carbon Soot and CO affect the human body from Incomplete Combustions?
Incomplete combustion of hydrocarbons produces carbon soot and carbon monoxide, both harmful to health. Carbon soot causes respiratory issues and can lead to lung cancer, while carbon monoxide reduces oxygen transport, causing symptoms like headache and nausea. Chronic exposure to carbon monoxide can lead to neurological and cardiovascular problems. Complete combustion is crucial to minimize the production of these harmful substances.
How does CO2 acidify the water
Carbon dioxide undergoes a synthesis reaction with water to form carbonic acid
Carbonic acid immediately breaks down in water, producing a hydrogen ions
This reaction of CO2 with water droplets in the atmosphere makes rain naturally mildly acidic
Normal rain = 5.6-7 on pH scale
How do nitrogen oxides contribute to the acidification of rain, and what is the primary source of nitrogen oxides in car exhaust?
Nitrogen oxides contribute to the acidification of rain by forming acids when they mix with water droplets. Most nitrogen in the atmosphere is stable and unreactive, but when air is drawn into car engines, high temperatures cause a synthesis reaction with oxygen (O2), producing nitrogen monoxide (N2O), also known as nitric oxide.
This compound is released into the atmosphere through car exhaust pipes and can cause respiratory irritation. Nitrogen monoxide mixes with water droplets to form nitric acid (HNO3), contributing to acid rain formation and smog formation.
How does Sulfur Oxide contribute to acid precipitation
S(s) + O2(g) → 2SO3,(g)
Once release into the atmosphere it sulfur dioxide mixes with additional oxygen to produce sulfur trioxide
2 SO3(g) + O2g) → 2SO3(g)
Sulfur trioxide combines with water in the atmosphere to form droplets of aqueous sulfuric acid
SO3(g) + H2O(l) → H2SO4(aq)
What are Metal Oxides
A binary compound formed between a metal and an oxide, these metal oxides are also known as basic oxides because they react with water to produce hydrides which are bases.
What are Nonmetal oxides
A binary compound formed between nonmetal and oxygen, these nonmetal oxides are known as acidic oxides because they can react with water to produce acids
What are Organic Compounds, Oxides, Acids and Bases
Organic compound → a molecular compound that contains one or more carbon-carbon bonds, and often, one or more carbon-hydrogen bonds
oxide = a compound composed of oxygen and one other element
acid = a compound that produces hydrogen ions when mixed with water, forming a solution that conducts electricity, tastes sour, turns blue litmus red, and neutralizes bases
base = a compound that produces hydroxide ions when mixed with water, forming a solution that conducts electricity, tastes bitter, turns red litmus blue, and neutralizes acids
Whats Qualitative and Quantitative Analysis
Qualitative analysis = the process of identifying substances present in a sample; no measurement involved
Quantitative = the process of measuring the quantity of a substance present in a sample. Providing numerical data
What is a mole and whats the avocados constant
Mole = a unit of amount of substance containing 6.02 x 1023 entities; unit symbol mol
Avogadro's constant (NA) the number of entities in 1 mol of a substance
What is Molar mass
Molar mass = the mass of 1 mol of a substance, unit symbol g/mol
The molar mass of a compound is the sum of the molar masses of each entity in the compound
The amount (n) and mass (M), of a pure substance are related to each other through the equation n= m/M
What is Empirical and Molecular Formul
Molecular = MF (the chemical formula that gives the actual number of atoms of each element)
Empirical = EF (the chemical formula that shows the lowest whole number ratio of each element)
What is the Law of definite proportions
law of definite proportions = that a given chemical compound always contains its component elements in fixed ratio and does not depend on its source and method of preparation
What is Stoichiometry
The process of determining the amount of one substance involved in a reaction using a given amount of another substance involved in the same reaction and the ratio between the two substances
When the ratio of the amounts (particles or moles) of two reactants matches the ratio of the balance coefficients in the equation, the reactants are said to be present in stoichiometric amounts. (equal amounts)
What are Limiting and Excess Reagents
limiting reagent the reactant that is completely consumed in a chemical reaction; the reactant that determines how much product will be formed. While excess reagent is a reactant that is still present after the reaction is complete.
What is theoretical yield and actual yield
TY (theoretical yield) = the amount of mass of product predicted based on the stoichiometry of the chemical equation
AY(actual yield) = the amount or mass of product actually collected during an experiment or industrial process.
Formula =Ay/Ty x 100% → (Percentage Yield)
What are Experimental Procedures and how do they effect the AY and TY
If some of the mass of a reactant is lost after it is measured, or some of the mass of the product is lost before it is measured, the AY will be less than the TY.
Causes
Spillage
Splattering
Isolation
What are Impurities and how do they impact AY and TY
The mass of the reactant in any sample of starting chemical is therefore less than the measured mass due to impurities.
What are competing side reactions and how do they affect AY and TY
Sometimes, competing reactions prevent some of the reactants from being converted into products.
For example, during the synthesis of cisplatin, the chemotherapy drug introduced in Section 7.3, a similar compound called transplatin is also formed
Define Solution, Solute, Solvent, Concentration, Dilute vs Concentrated Solution, Alloy, Amalgam and Aqueous Solution
Solution: A solution is a homogeneous mixture of two or more substances.
Solute: A solute is a substance dissolved in a solvent.
Solvent: A solvent is a substance that dissolves the solute.
Concentration: Concentration is the amount of solute in a given quantity of solvent or solution.
Dilute vs Concentrated Solution
Dilute solution: Contains a small amount of solute.
Concentrated solution: Contains a large amount of solute.
Alloy: An alloy is a mixture of two or more elements, with at least one being a metal.
Amalgam;An amalgam is an alloy containing mercury.
Aqueous Solution: An aqueous solution is a solution where water is the solvent.
Define Hydration, Dissociation, Miscible and Immiscible
Hydration = the process in which ions are surrounded by water molecules
Dissociation = the separation of individuals ions from an ionic compound as it dissolves in water
Miscible = able to mix to form a solution; usually describing liquids that mix with each other in all proportions to form a solutions
Immiscible = unable to mi to form a solution; unusual describing liquids that do not readily mix
“Like dissolves Like” = solutes dissolves in solvent if similar polarity
Define Saturated Solution, Unsaturated Solution, Supersaturated Solution and Solubility Curve
saturated solution = a solution that contains the maximum quantity of solute at a given temperature and pressure
unsaturated solution = a solution in which more solute can dissolve at a given temperature and pressure
supersaturated solution = a solution that contains more than the maximum quantity of solute that it should at a given temperature and pressure
Solubility curve = a graph of the solubility of a substance over a range of temperature
What is Molar and Standard Solutions
Molar concentration = An amount concentration in moles is divided by the volume of the solution in liter
Standard Solution = a solution whose concentration is known both precisely and accurately
What is a Formula Equation and Net Ionic Equation
Formula equation: a chemical equation in which all compounds are represented by their chemical formulas
Total Ionic equation: a chemical equation in which all highly soluble ionic compounds are written as dissociated ions
What are Spectator Ions and Net Ionic Equation =
Spectator ion: ions that are no involved in a chemical reactions
Net ionic equation: a chemical equation that indicates only the entities that react during the reaction
Know this
Flowcharts based on Solubility and change in chemical state
Bonus - Boyle’s Law, Charles's Law, Gay-Lussac’s Law, Avogadro’s Law and Daltons Law of Partial pressures
Boyle - The volume of a given amount of gas at a constant temperature varies inversely with the applied pressure
Charles - The volume of a given amount of gas at a constant pressure is directly proportional to its kelvin temperature
Gays Law - The pressure of a given amount of gas at a constant volume is directly proportional to its kelvin temperature
Avogadro’s law - the volume of a gas is directly proportional to the amount of the gas when the temperature and pressure are held constant
Dalton’s Law - The total pressure exerted by a mixture of non-reacting gases is equal to the sum of the partial pressures of each gas