Excited States + Terms & States
1/97
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
98 Terms
Photochemistry
Chemistry initiated by light.
Electronic Transition
When an electron moves from one molecular orbital to another.
Chromophores
Molecules which absorb light.
Photophysics
The non-reactive decay of excited states.
Photochemistry
The reactive decay of excited states.
Ground State
The state which most molecules occupy, where electrons usually occupy bonding and non-bonding MOs.
Excited State
When a molecule absorbs a photon, causing an electron to transition to a higher energy MO.
How do you name an excited state?
Names after the orbitals with one electron
Eg. π,π*
The full name includes the multiplicity
Eg. 1(π,π*) or 3(π,π*)
What is the equation for multiplicity?
2S+1
Where S is the total spin quantum number, given by:
1/2(no. of e- with positive spin) - 1/2(no. of e- with negative spin)
Hund’s Rule
Unpaired electrons in orbitals have the same spin - hence a triplet state is lower in energy than the equivalent singlet state
Born-Oppenheimer Approximation
Total wavefunction is the product of the electronic, vibrational, and rotational wavefunctions.
ψtotal = ψe ∙ ψv ∙ ψr
Etotal = Ee + Ev + Er
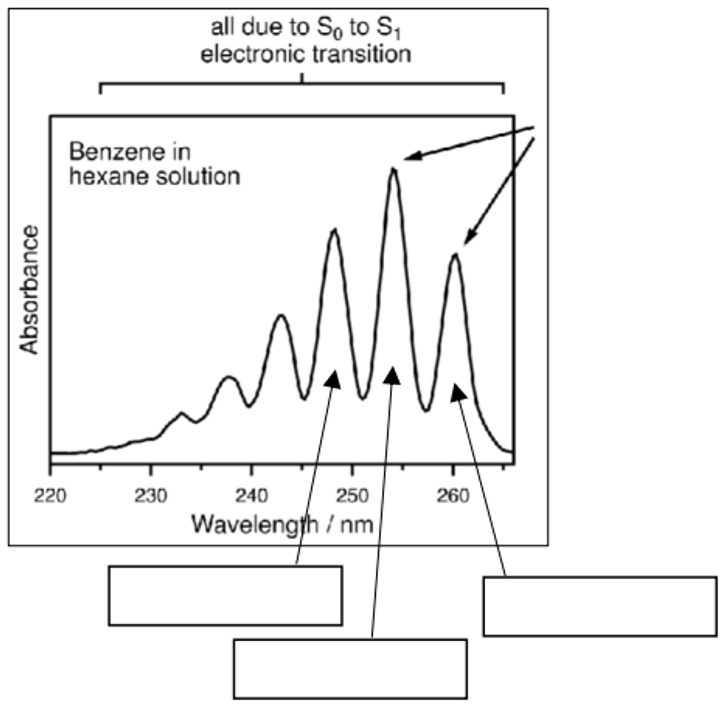
What are the transitions labelled by the arrows?
Overall Selection Rule Equation
Demonstrates that in order to excite an electron, there must be a displacement of charge which gives a transition dipole moment.
What are the three selection rules?
Spin, orbital, vibrational
Spin Selection Rule
A transition is spin disallowed if there is a change in state multiplicity.
How can the spin selection rule be defined in terms of ∆S?
∆S is the change in multiplicity:
If ∆S = 0, the transition is S0 —> S1, so spin allowed
If ∆S ≠ 0, the transition is S0 —> T1, so spin disallowed
What impact does the presence of heavy atoms have on the spin selection rule?
Spin flip transition are less disallowed in the presence of heavy atoms (in the molecule or solvent).
This is because there is increased spin-orbit coupling.
This means that the transfer of spin angular momentum to orbit angular momentum is more likely, so these transitions are more likely.
Orbital Selection Rule - Symmetry component
If any one of the three direct products has A1/Ag (highest symmetry of the point group) then it is allowed.
What is the symmetry of a spin state with all electrons paired?
Totally symmetric - A1 or Ag
(highest symmetry of point group)
What is the symmetry of a spin state with unpaired electrons?
The product of the symmetries of the unpaired electron orbitals
(symmetries of orbitals for this given in this course)
What is the equation to give the 3 symmetry products in the orbital selection rule?
Гi x Гx,y,z x Гf

How do you find Гx,y,z ?
Find x, y or z in the right hand column of the point group

What does this mean and what can be said about this transition?
This transition is symmetry allowed.
It is polarised along the z axis.
Spatial Component of Orbital Selection Rule
Dictates the magnitude of the orbital component based on the spatial overlap of orbitals.
Good overlap gives a larger integral (aka spatially allowed)
Vibrational Selection Rule
The magnitude of a transition will be larger for transitions with large vibrational overlap integrals.
How does the S1 equilibrium bond length differ from that of S0 and why?
Often longer because an electron has been promoted to an antibonding orbital, which weakens the bonds
What does it mean in terms of vibrational overlap integrals if re(S1) = re(S0)
If the equilibrium bond lengths are the same for S1 and S0 then the highest intensity transition will be the v”=0 —> v’=0
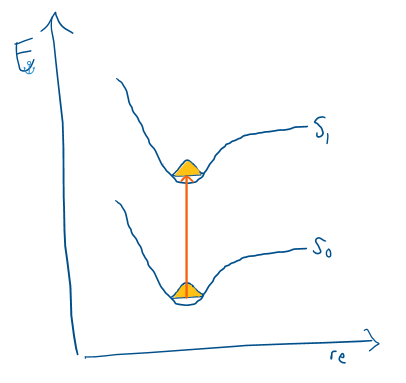
Franck-Condon Principle
The position of the atoms is unchanged during the transition because electrons move faster than nuclei.
Therefore, any change in geometry occurs after the electronic transition
Oscillator Strength (f)
Represents the probability of a transition.
Comprised of spin and orbital (symmetry and spatial) components
An f value of 1 denotes a fully allowed transition
Which selection rule has the largest impact on oscillator strength (probability of transition)?
Spin
Fluorescence
A radiative transition to a state of the same multiplicity (S1→S0).
What are the similarities and differences between an absorption spectrum and a fluorescence spectrum?
Similarities:
Both show vibrational fine structure
Both absorption and fluorescence mostly occur from the lowest vibrational energy level (due to Boltzman and vibrational relaxation)
Differences:
Absorption spectra show the fine structure of the excited states vibrational levels, fluorescence spectra show the fine structure of the ground state
How can you observe fluorescence from v’>0 states?
Record the spectra of the compound in the gas phase.
There’s fewer collisions so the rate of vibrational relaxation is lower
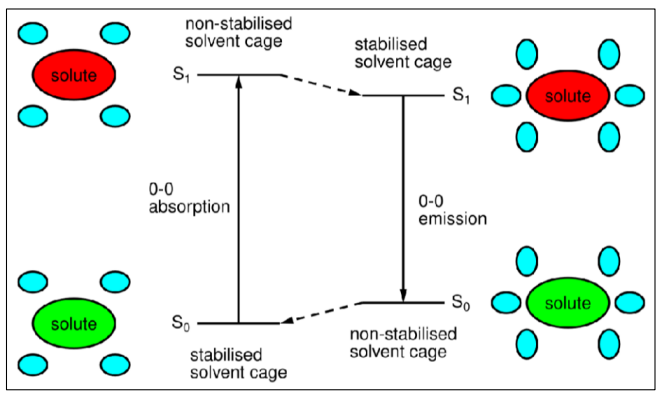
Why is the wavelength of absorbance and fluorescence spectra slightly different?
The S1/S0 energy gap becomes smaller in the excited state.
The solvent reorganises to stabilise the excited state, which slightly destabilises the ground state
Phosphorescence
A radiative transition between states of different multiplicity (T1 → S0).
(By definition, spin disallowed - slow)
How does a phosphorescence spectrum compare to a fluorescence spectrum?
They both show excited state v’=0 —> transitions, but phosphorescence spectra are at longer wavelengths.
This is because the triplet state is lower in energy due to Hund’s rule (unpaired electrons favour having the same spin)
Vibrational Relaxation (vr)
Excess vibrational energy is given to surrounding molecules by collisions.
What is the rate of vibrational relaxation dependent on?
The rate of collision
(very fast, occurs before anything else)
Internal Conversion (ic)
A non-radiative transition between states of the same multiplicity.
Draw a diagram to depict internal conversion
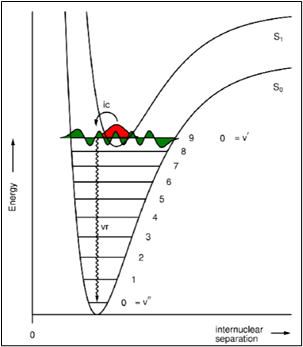
Kasha's Rule
vr and ic happen so rapidly between (different) excited states that fluorescence only occurs from the S1 state.
Eg. S3 —> S2 —> S1 very rapid,
S1 —> S0 slower (due to larger energy gap) so fluorescence can occur
Intersystem Crossing (isc)
A non-radiative transition between states of different multiplicity.
Explain why T1 states can be fairly long lived
isc is spin disallowed, but isc’ is even slower due to the large T1/S0 energy gap
Jablonski Diagrams
Energy states displayed as stacks, with energy as the vertical axis.
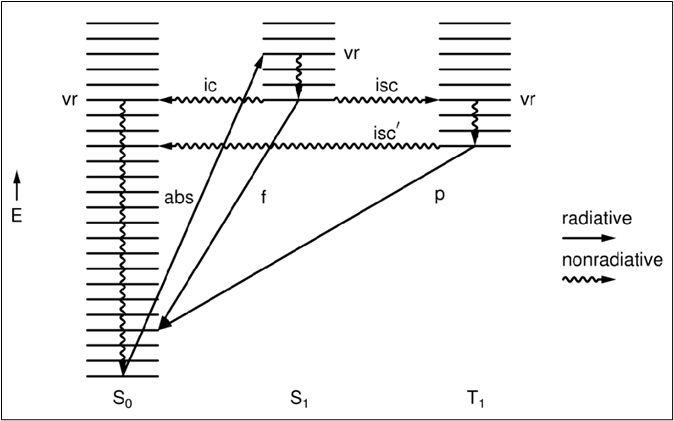
Chemiluminescence
Excitation caused by a chemical reaction.
Quantum Yield
The number of molecules undergoing a process / the number of photons absorbed.
OR
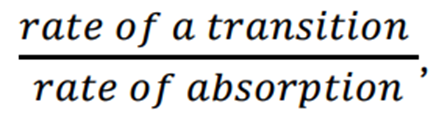
Write the general equation for the quantum yield of fluorescence?
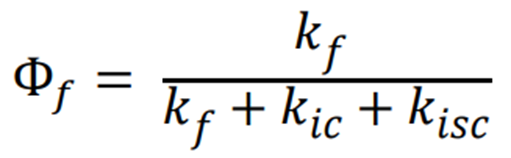
Quantum Efficiency
The quantum yield of transitions from the T1 state
Lifetimes
When [A*] falls to 1/e of its initial value.
What is the equation for lifetime?
τ = 1/k
What impact does temperature have on photophysical processes?
Temperature doesn’t impact photophysical rates.
It can impact competing processes, such as isomerisation
Draw a diagram to depict how Q can act as an electron acceptor in exciplexes
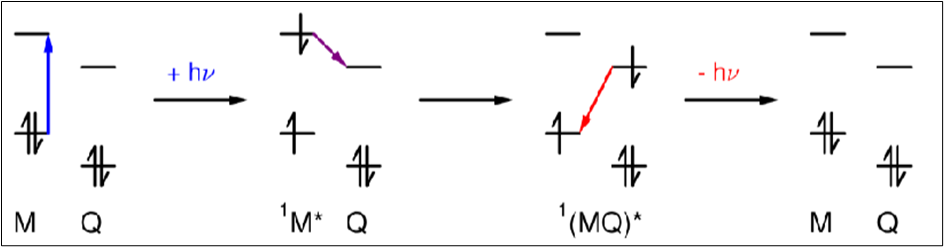
Draw a diagram to depict how Q can act as an electron donor in exciplexes
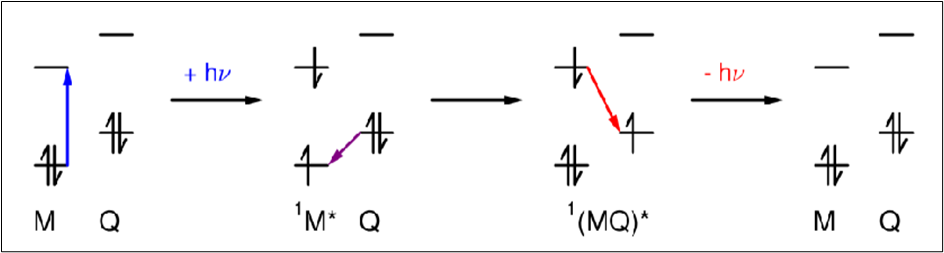
Quenchers
Substances in solution that collide and cause T1 states to decay.
How do you decrease the impact of quenchers? What impact does this have?
Turn the solution into a rigid matrix (solid).
This stops the quencher from being able to diffuse to collide.
Therefore, photophysical decay dominates and phosphorescence is more visible.
Heavy Atom Effect
The presence of heavy atoms in a molecule increases the rate of all ‘spin-flip’ processes.
This is due to an increase in spin-orbit coupling.
What are the two types of experimental methods?
Steady-state techniques (measuring the wavelength of emission/absorbance upon irradiation (at 90 degrees))
Time-resolved techniques (light pulse)
Photochemistry
The reactive decay of excited states
How does excitation change the reactivity of a molecule?
Changes electronic configuration
Gain of energy to overcome activation barriers
Formation of long-lived T1 states which can then react
Photodissociation (give equation)
When a molecule dissociates upon excitation.
AB + hv → AB* → A + B
What is the dissociation limit? Draw a diagram
When the absorbance of a wavelength above a certain value causes the molecule to dissociate.
Above this limit, there is no vibrational fine structure in the absorbance spectrum because the absorbance becomes continuous
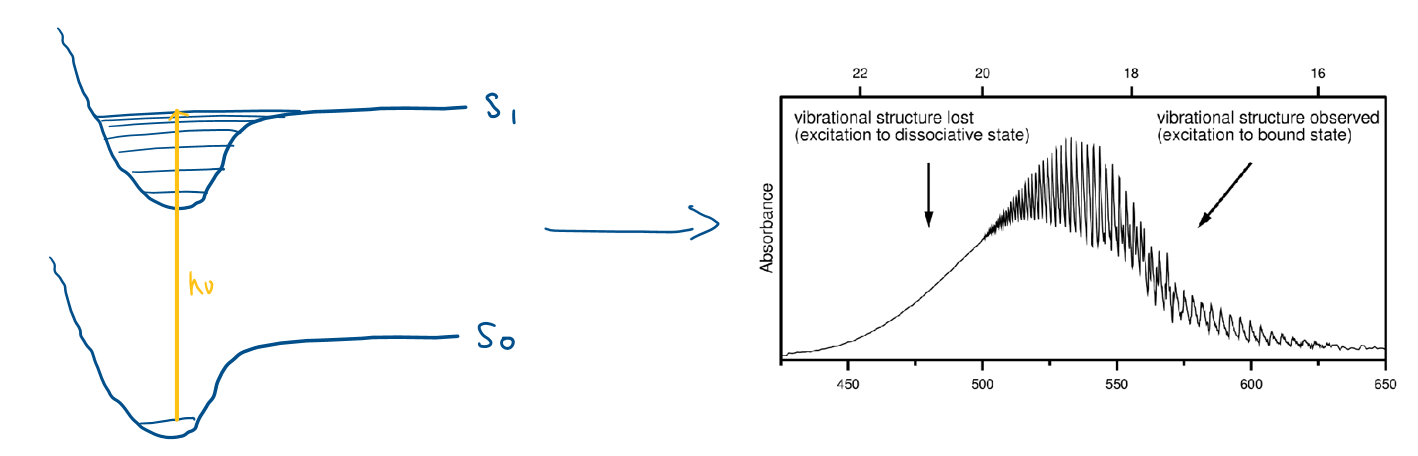
What is the difference between photodissociation in the gas phase and in solution/liquid phase?
In the gas phase, the fragments move freely apart upon dissociation.
In the liquid phase (/solution), the fragments may be held together long enough by the solvent cage to recombine.
Photopredissociation
Excitation causes a vibrationally excited state to form, which internally converts rapidly into a dissociative state.
Vibrational fine structure is lost in the absorbance spectrum at the wavelength which rapidly converts into the dissociative state
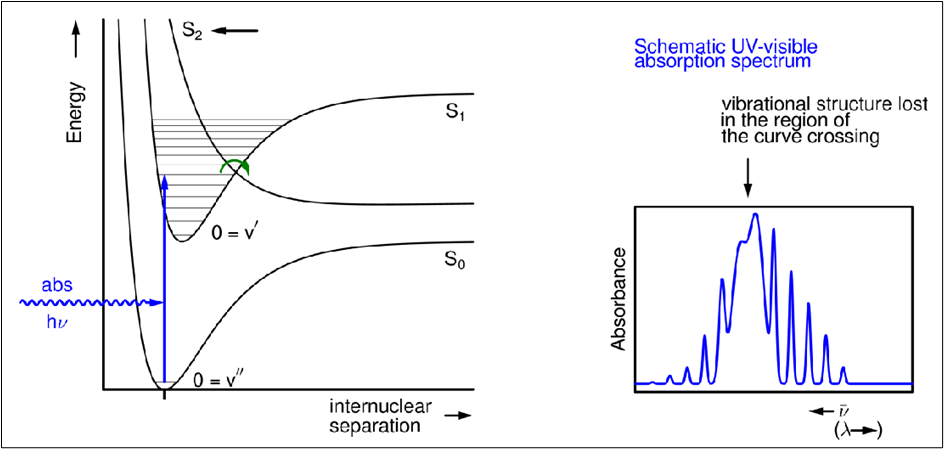
Photoisomerisation (give equation)
When a molecule changes structure (eg. isomerises) upon excitation.
AB + hv → AB* → AB’
Describe photoisomerism in alkenes
Excitation causes the alkene to form its twisted structure.
This can then decay into either the cis or trans isomer.
If you only irradiate with the wavelength which excites one of the isomers, you can get up to 100% conversion into the other.
The balance of isomers produced under irradiation is called the photostationary state.
Photochromism
A change in color upon excitation.
Generally caused by isomerisation (cis/trans) or ring opening/closing.
Excimers (give equation)
A system which produces a dimer in the excited state.
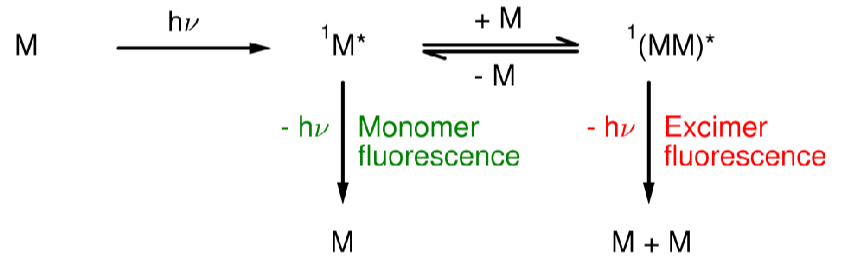
When does excimer fluorescence occur?
When [M] is high
How can you identify excimer fluorescence experimentally?
An excimer fluorescence peak will be at lower energy and won’t show vibrational fine structure
Recording fluorescence spectra at different [M] - The excimer peak will decrease in intensity at lower [M]
How does the emission intensity of a monomer over time compare to that of an excimer?
A monomer will decrease over time after excitation.
An excimer will increase initially as the excimer forms, then begin to decay
Exciplexes (give equation)
A system which forms a complex upon excitation.
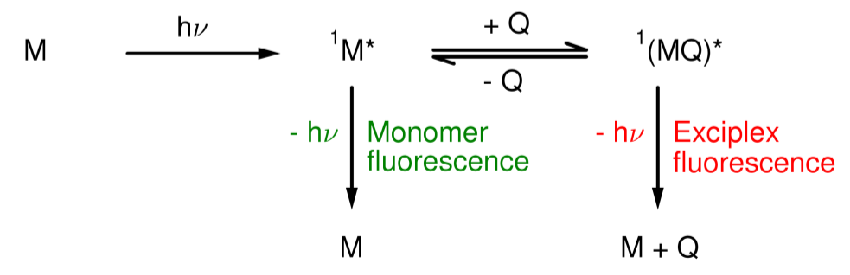
Draw a diagram to depict monomer and exciplex/excimer emission and the cause of them being at different wavelengths
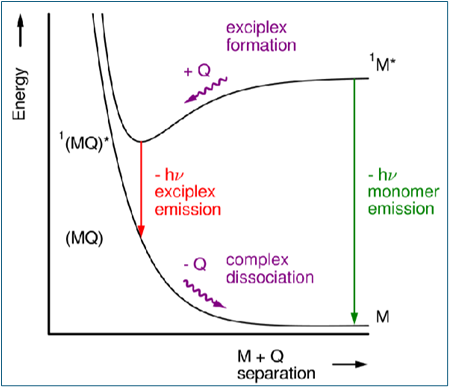
What is the main difference between excimers and exciplexes?
In exciplexes, there is a significant degree of charge transfer between M and Q
What is τ0obs in the Stern-Volmer equation?
The observed lifetime without a quencher
Diffusion Controlled Limit
The rate of quenching is controlled by the rate of diffusion of the quencher to the molecule.
What is the rate of diffusion dependent on?
Temperature: higher temperature = faster diffusion
Viscosity: higher viscosity = slower diffusion
Electron transfer
Quenching via an electron transfer between M* and Q
Draw a diagram to depict Q acting as an electron acceptor in electron transfer quenching. What type of quenching is this?
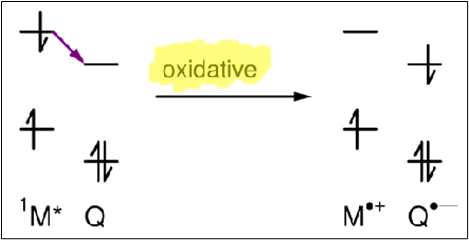
Draw a diagram to depict Q acting as an electron donor in electron transfer quenching. What type of quenching is this?
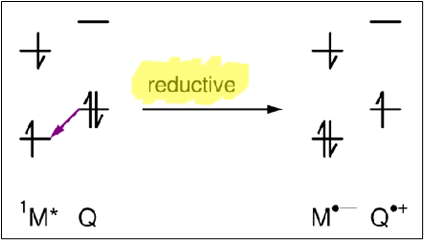
What impact does solvent polarity have on electron transfer quenching?
In a low polarity solvent, the exciplex is more energetically stable.
In a high polarity solvent, the ion products are energetically favoured because they are stabilised by the solvent
Energy Transfer
The transfer of electronic excitation energy from a donor to an acceptor molecule.
Any further reaction of the acceptor is called ‘sensitised’
What are the 3 mechanisms of energy transfer?
Radiative
Non-radiative: long-range Coulombic, short-range electron exchange
Write an equation to depict radiative energy transfer
D* —> D + hv
A + hv —> A*
When does radiative energy transfer dominate?
When the solutions are in low concentration,
or when the molecules are far apart eg. Sun —> Earth
Long-Range Coulombic Energy Transfer
Energy transfer by a dipole-dipole interaction between D* and A, no contact or photon emission involved.
Draw a diagram to depict long-range Coulombic energy transfer
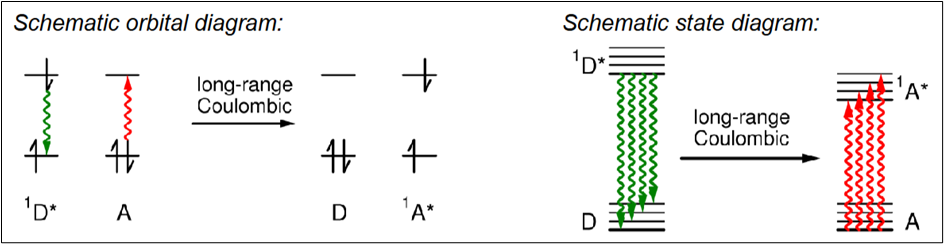
What are the requirements for long-range Coulombic energy transfer?
D/D* and A/A* energy gaps must be the same
D* and A must be 2-10nm apart
Short-Range Electron Exchange Energy Transfer
Energy transfer by the exchange of electrons between D* and A, no photon emission involved.
Draw a diagram to depict short-range electron exchange
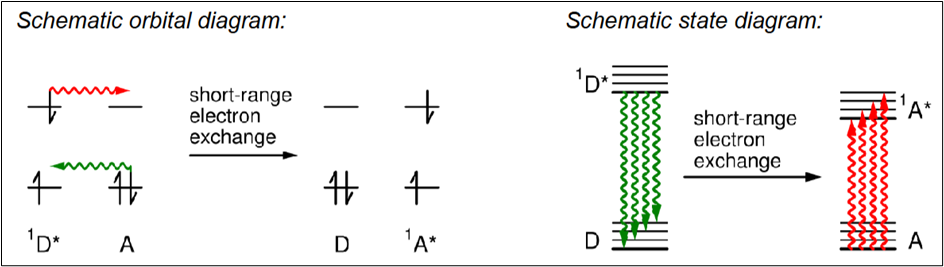
What are the requirements for short range electron exchange?
D/D* and A/A* energy gap must be the same
D* and A must be about 1 nm apart
Photosensitisation (give equation)
Triplet states can be produced by triplet-triplet electron exchange
T1 + S0 —> S0 + T1
What is the equation for spin-orbit coupling?
J = L + S
How do electrons interact?
The orbital angular momentum of the two electrons are coupled because they repel each other.
The strength of this interaction is measured through the Racah parameter (B)
What is the general form of a term symbol?
2S+1L
What are the letters given for these L values?
A) L = 0
B) L = 1
C) L = 2
D) L = 3
A) S
B) P
C) D
D) F
What are the mL and mS values for these microstates?
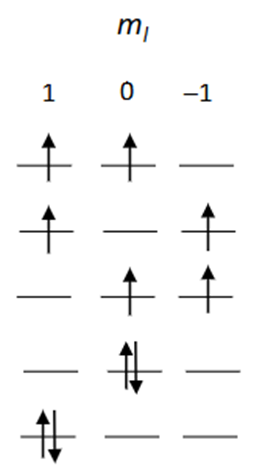
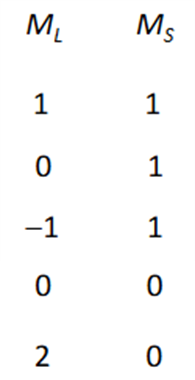
Draw the ground state for an element with p2 valence electrons. Explain why this is the ground state.
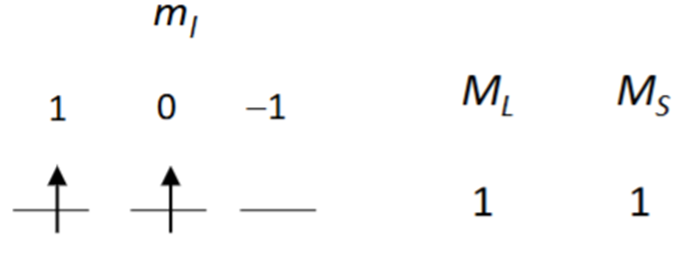
Hund’s rules state that the ground state has maximum spin and orbital angular momentum
What are the steps to determining the term symbols for an electronic configuration?
1) Find the ground state term symbol