IB Biology- C1.1: Enzymes and metabolism
1/51
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
52 Terms
What is metabolism?
The sum of all chemical reactions taking place in a cell at one time.
What does it mean that metabolic pathways are interdependent?
They rely on each other to occur. e.g. A+B=C then C+D=E
What is anabolism and some examples?
The joining together of monomers to form complex molecules in condensation reactions. It requires energy from ATP to occur.
Some examples of this are protein synthesis, glycogen formation, and photosynthesis.
What is catabolism and some examples?
Catabolism is the breaking apart of complex molecules into smaller components in a hydrolysis reaction. It releases energy as it occurs.
Some examples of this are digestion and oxidation of substances in respiration.
What are 4 different forms of energy that are important to organisms?
1. Kinetic energy: energy of motion
2. Potential energy: stored energy/energy in a form that isn't being used at a point of time
3. Chemical energy: potential energy available for release when a chemical reaction occurs
4. Thermal energy: a form of kinetic energy stored within objects. It is capable of being transferred from one object to another as heat.
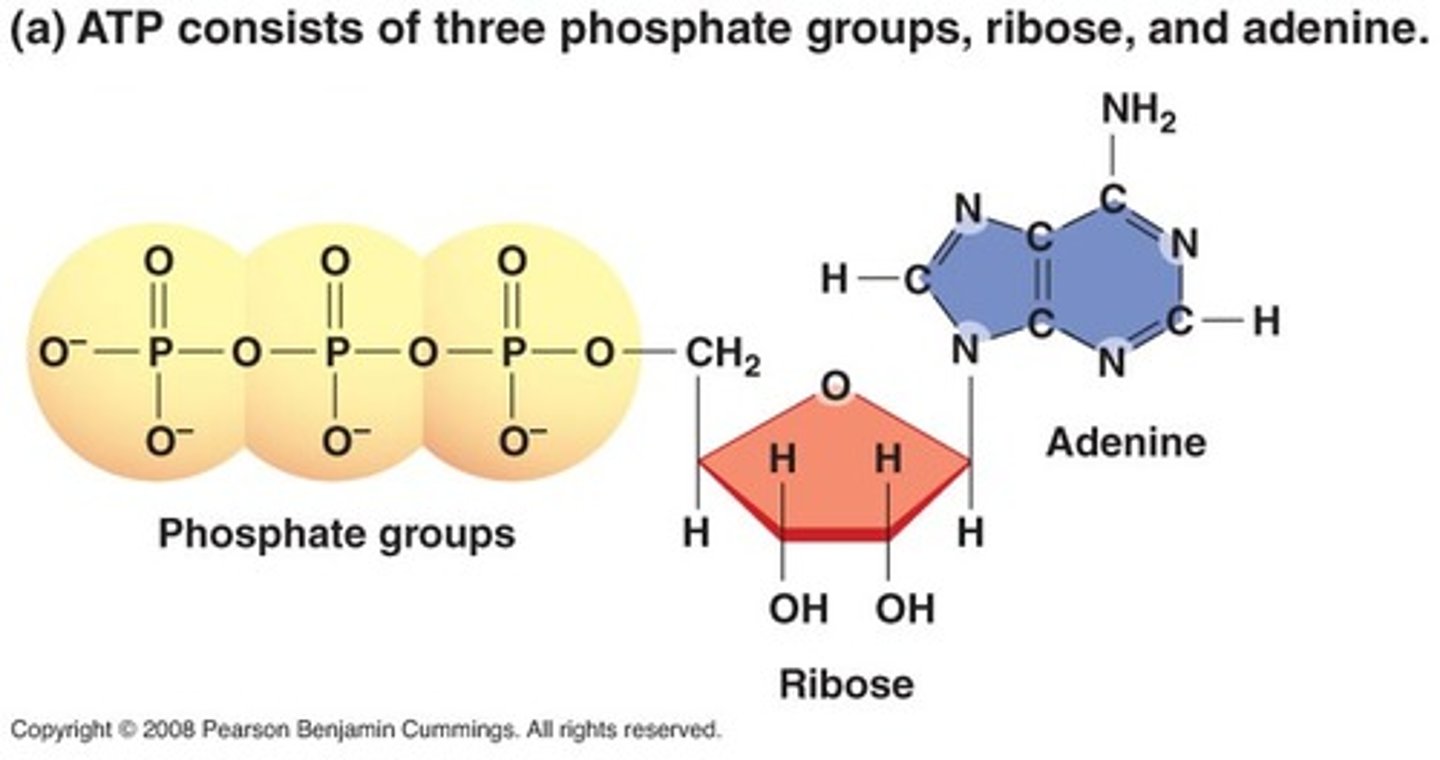
What is the structure and function of ATP (adenosine triphosphate)?
Adenosine triphosphate:
- Supplies the energy needed to synthesise macromolecules
- Supplies the energy needed for mechanical work e.g. muscle action
- Provides energy to move substances across the cell membrane e.g. sodium-potassium pump
Why are many different enzymes needed in the body?
Enzymes are highly specific so different enzymes are needed to bind to different proteins and carry out different chemical reactions.
What are enzymes and what is their function?
Enzymes are globular, 3D proteins and compact molecules. They are biological catalysts, speeding up the rate of already existing chemical reactions in the cell by lowering the activation energy needed for them to take place and are NOT used up in the process. They have specific active sites where a substrate binds.
What is the benefit of enzymes?
Enzymes force a collision between molecules which speeds up the reaction. This is useful because it allows reactions to occur at a fast enough rate to sustain life but at lower temperatures and lower energy inputs that they would need otherwise. Essentially, they increase the rate of reaction without raising temperatures (as this would damage and denature proteins). Also, if we didn't have enzymes we would constantly be eating to supply enough glucose for respiration.
What is needed for a substrate molecule and active site to come together?
Movement
What is activation energy?
The energy input required to start a reaction, much of which comes from the collisions of the reactants.
How do enzymes bind to substrates?
They have an active site which is complementary to the substrate molecule.
What is an active site?
A small indentation on the surface of the enzyme, forming a hollow depression in the molecule. The substrate molecule fits into this depression to form a enzyme-substrate complex and temporary bonds form between the substrate and certain amino acids of the active site.
How much of the enzyme molecule is involved in binding to the substrate?
Only a small number of the amino acids of the enzyme are significant in forming bonds with the substrate; however other sites on the molecule are responsible activating the enzyme or preventing the active site from working.
What are the two models for enzyme-substrate binding?
- Lock in key
- Induced fit/Hand in glove
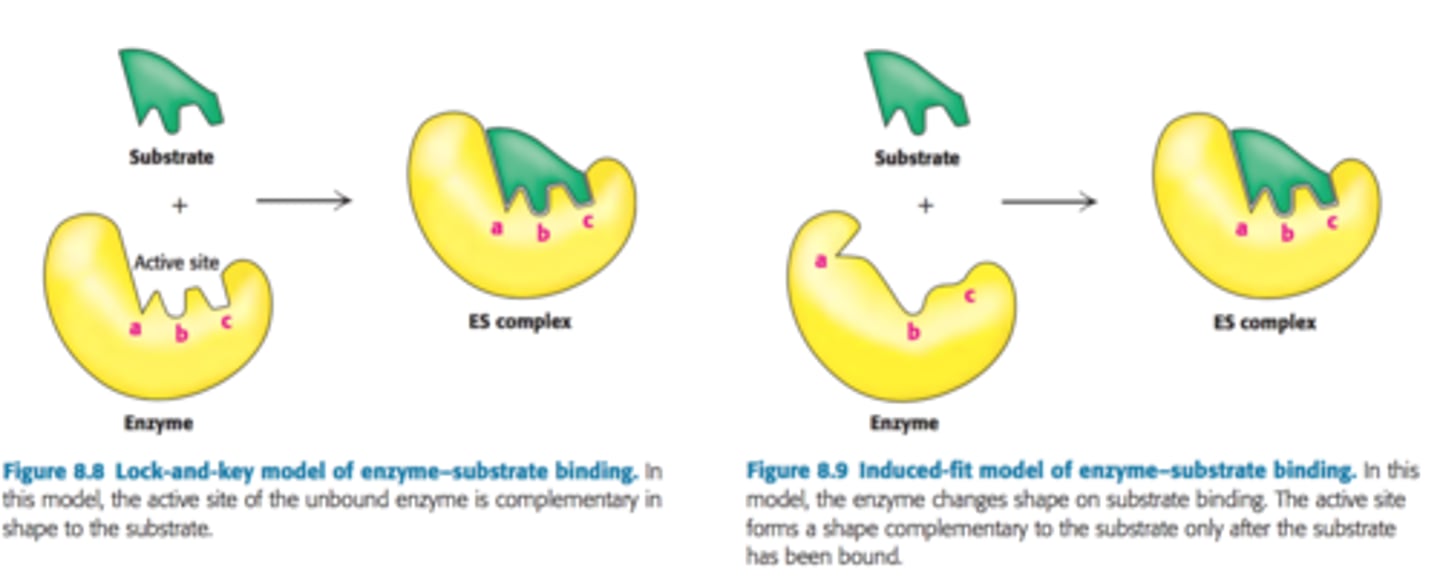
What is the most accepted model for substrate-enzyme binding?
The induced fit model
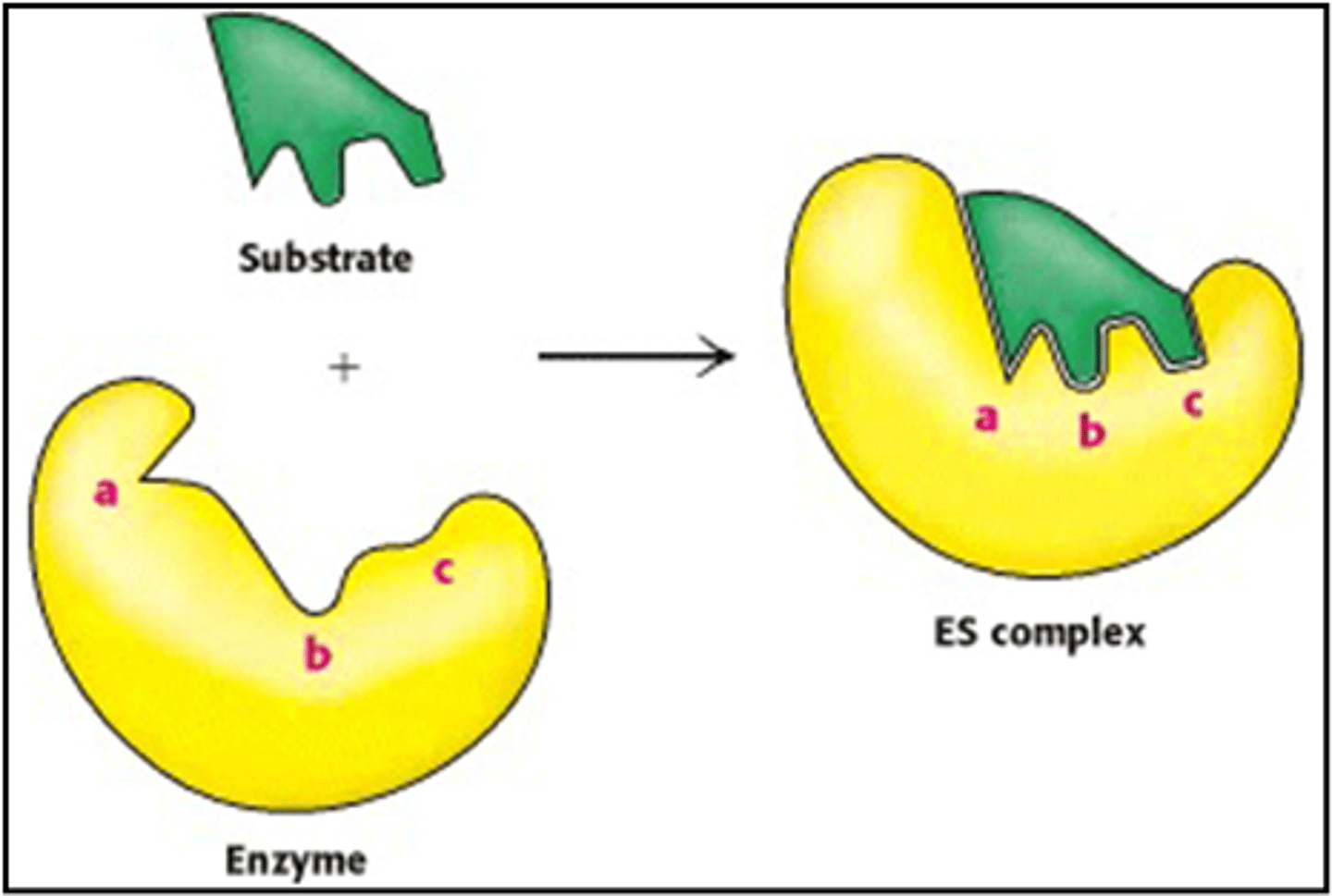
Why is this more widely accepted?
It shows how the enzyme and substrate are flexible and slightly change shape as the enzyme-substrate complex is formed. It therefore implies that the substrate isn't perfectly complimentary but that it changes shape slightly to accomodate the substrate.
Summary of the mechanisms of enzyme action:
1. The surface of the substrate makes contact with the active site of the enzyme.
2. The enzyme and substrate change shape, forming a temporary enzyme-substrate complex.
3. The activation energy is lowered and the substrate is altered by the rearrangement of the existing atoms.
4. The transformed substrate, the product, is released from the active site.
5. The unchanged enzyme is free to combine with other substrate molecules.
How does an enzyme actually work?
As the enzyme and substrate slighlty change shape, the enzyme exerts stress on the bonds between the substrate molecules, weakening them and meaning that the substrate molecule requires less energy to break these bonds and form the products. The opposite happens when molecules are forming- the enzyme pushes them together to encourage a bond to be formed. As such enzymes lower the amount of activation energy needed for a reaction.
What does a graph of activation energy with an enzyme VS without an enzyme look like?
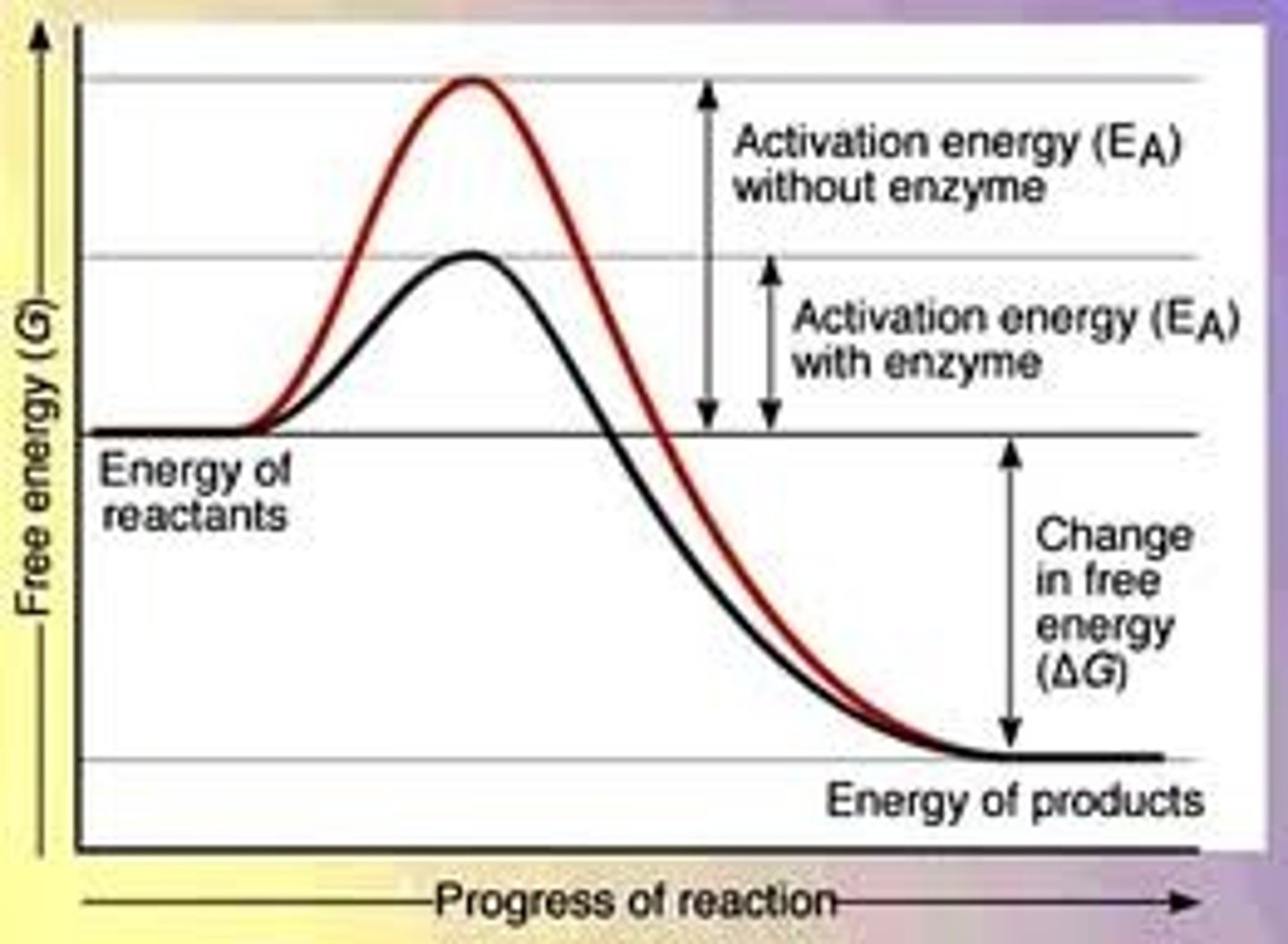
What factors affect the rate of enzyme-controlled reactions?
- Temperature
- pH
- Substrate concentration
- Enzyme concentration
How to 'describe' a graph?
- Use phrases such as 'Between x and y on the graph' and 'At x degrees C'
- Use adjectives to describe the line e.g. gradual, steep
- Talk in terms of their words (labels on the axes)
How can rate be measured?
The amount of product produced or the substrate breakdown in a given length of time (per second/per minute)- dy/dx.
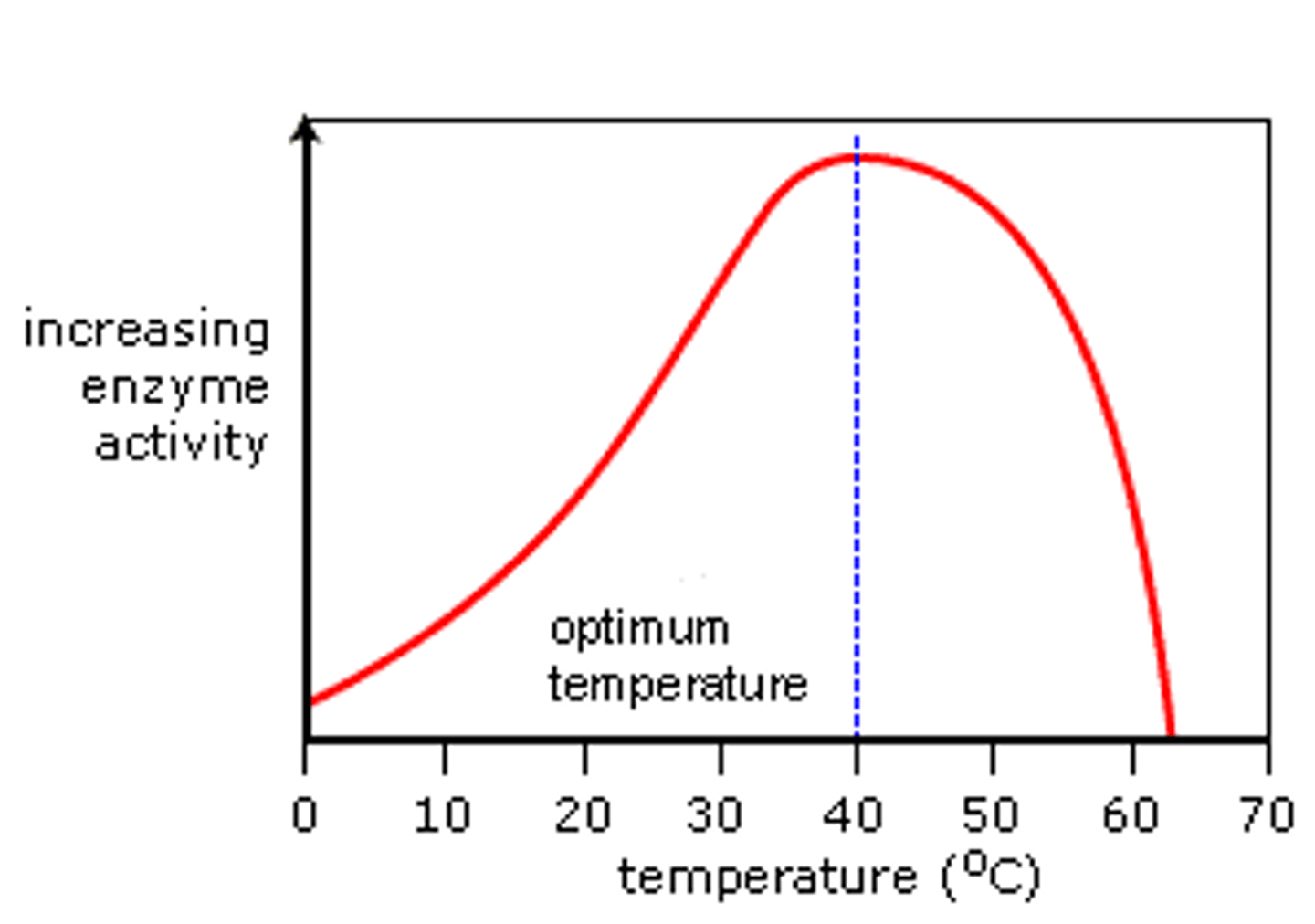
How does temperature affect the rate of enzyme-controlled reactions?
As the temperature rises, the substrate molecules gain more energy in their kinetic energy stores and so move more quickly, colliding more frequently and with more power with the active site of the enzymes, thus increasing the rate of reaction. When the enzymes reach their optimum temperature, their activity is at its peak. After this, the high temperatures cause the active site to change shape and denature as the intramolecular hydrogen and ionic bonds begin to break, changing its tertiary structure, therefore the rate of enzyme activity decreases until finally the substrate molecule can no longer bind to the active site and no enzyme-substrate complexes are formed.
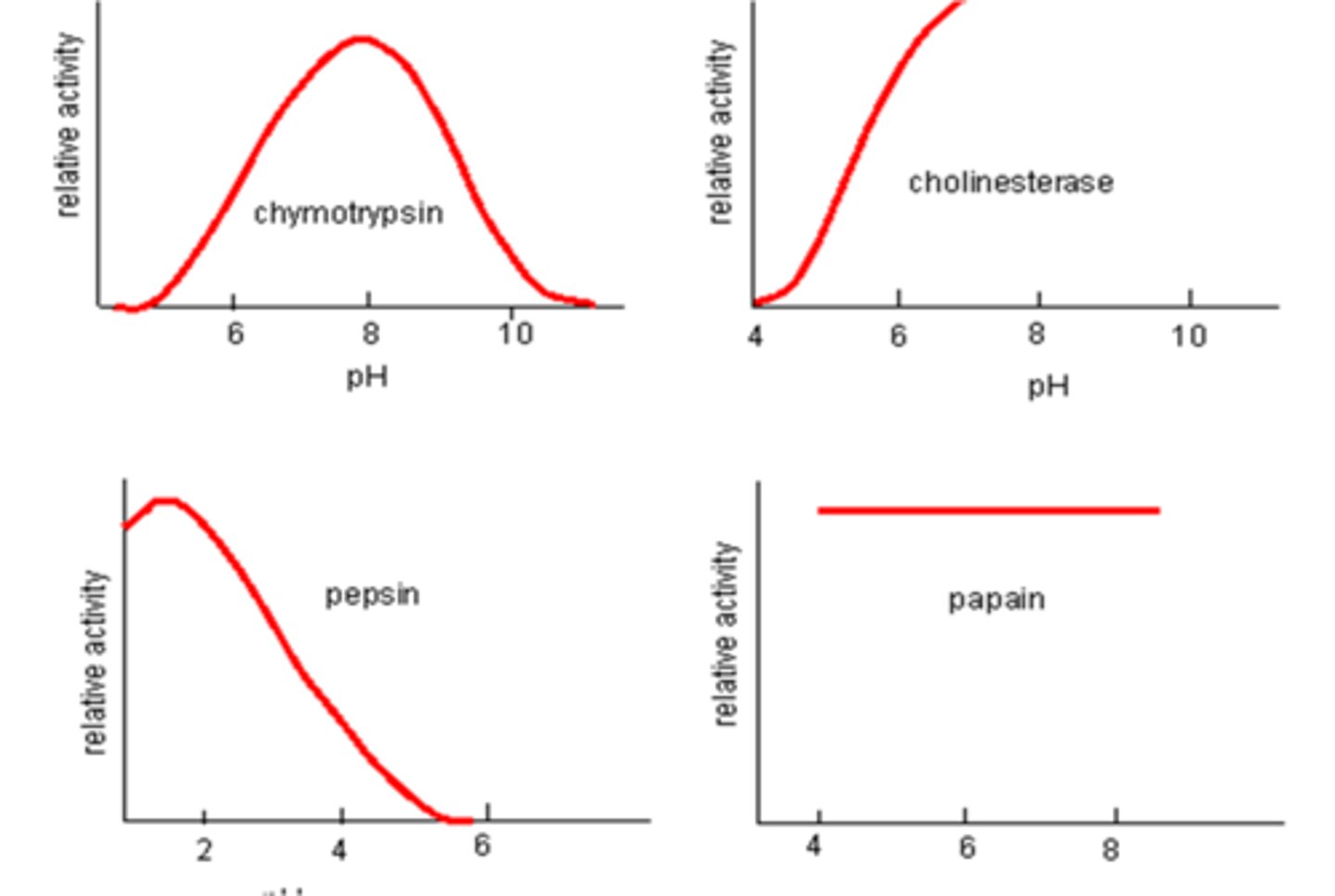
How does pH affect the rate of enzyme-controlled reactions?
An active site of an enzyme must have the same charge as the charged areas of the substrate that bind to it. If pH is too acidic or basic, this can interfere with these charges and even cause the bonds between the proteins in the enzyme to break, denaturing it. Often enzymes work in quite a narrow pH range but this differs between enzymes as they work in different environments e.g. enzymes in the stomach will work in acidic conditions whereas enzyme in bile are alkaline
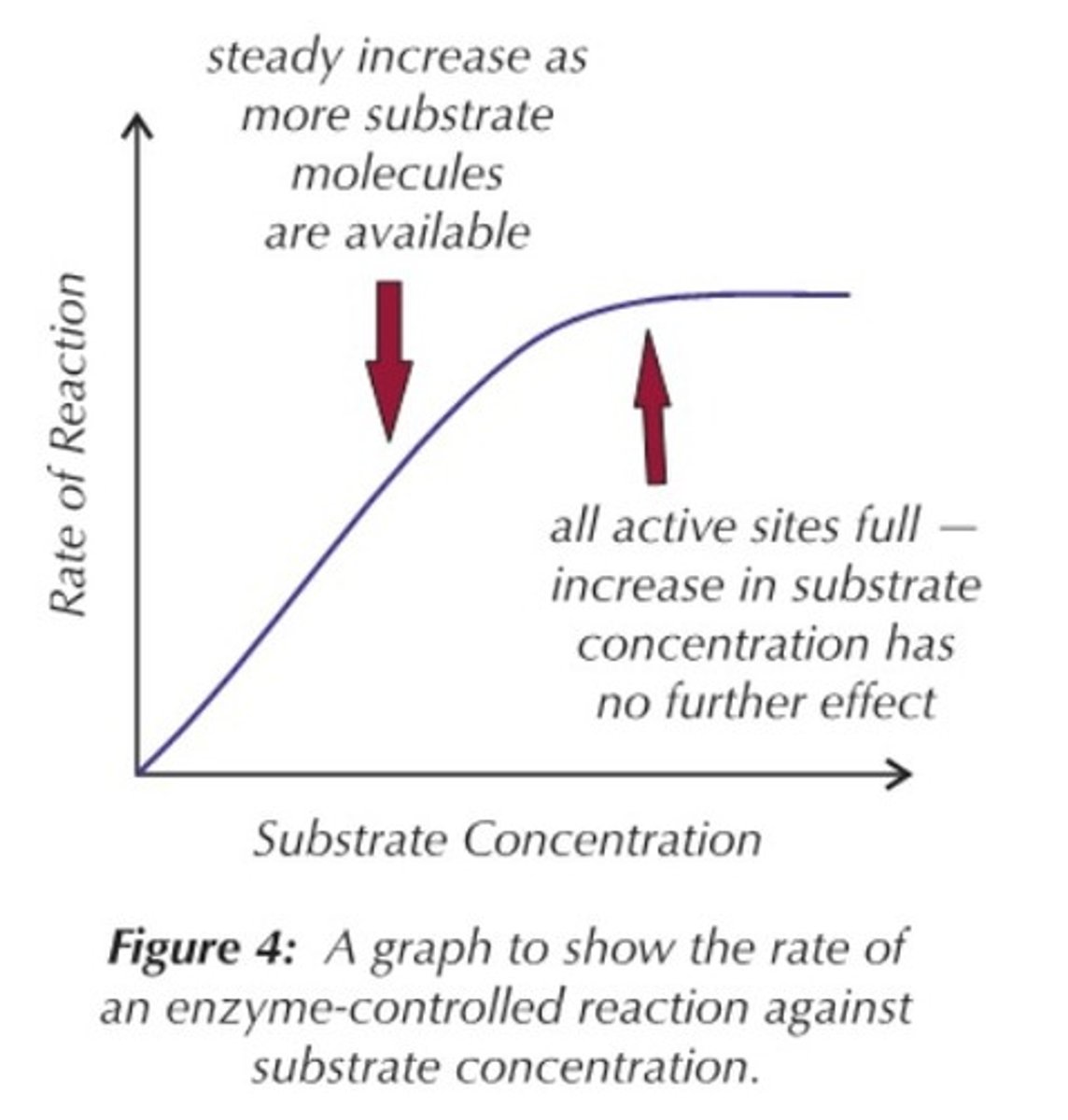
How does substrate concentration affect the rate of enzyme-controlled reactions?
As substrate level increases, the rate of reaction also increases. It does this to a certain point at which the rate of reaction will reach a maximum rate and stop increasing because there would not be enough enzyme to catalyse the reaction and this would now become the limiting factor. All the active sites of the enzymes are saturated (occupied), meaning they can't work any faster.
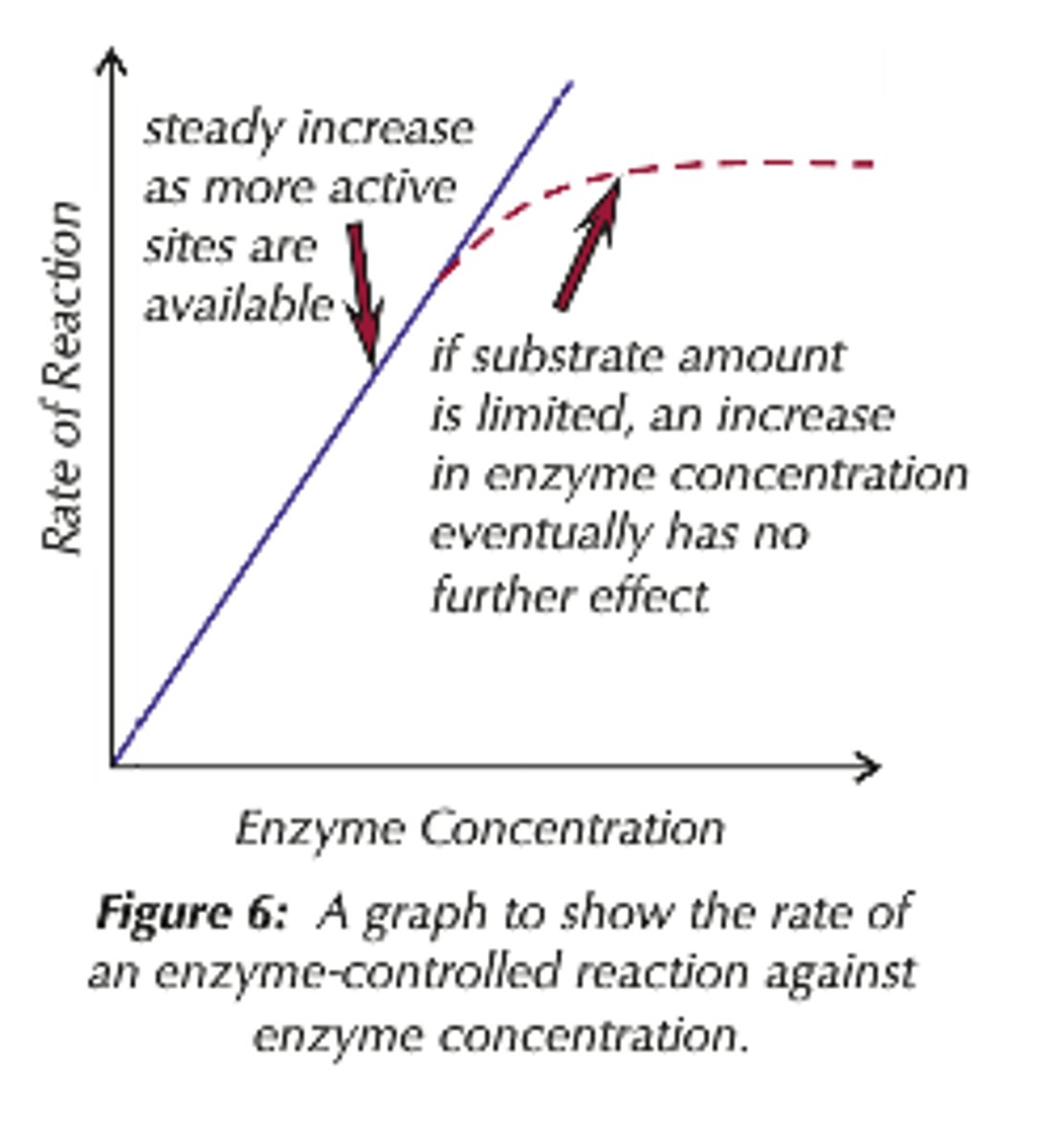
How does enzyme concentration affect the rate of enzyme-controlled reactions?
As enzyme level increases, the rate of reaction also increases. It does this to a certain point at which the rate of reaction will reach a maximum rate and stop increasing because there would not be enough substrate to for enzyme-substrate complexes with the enzyme so the substrate would now become the limiting factor.
What happens when bonds are broken?
Energy is taken in from the surroundings (endothermic). Therefore the temperature of the products is higher than the reactants.
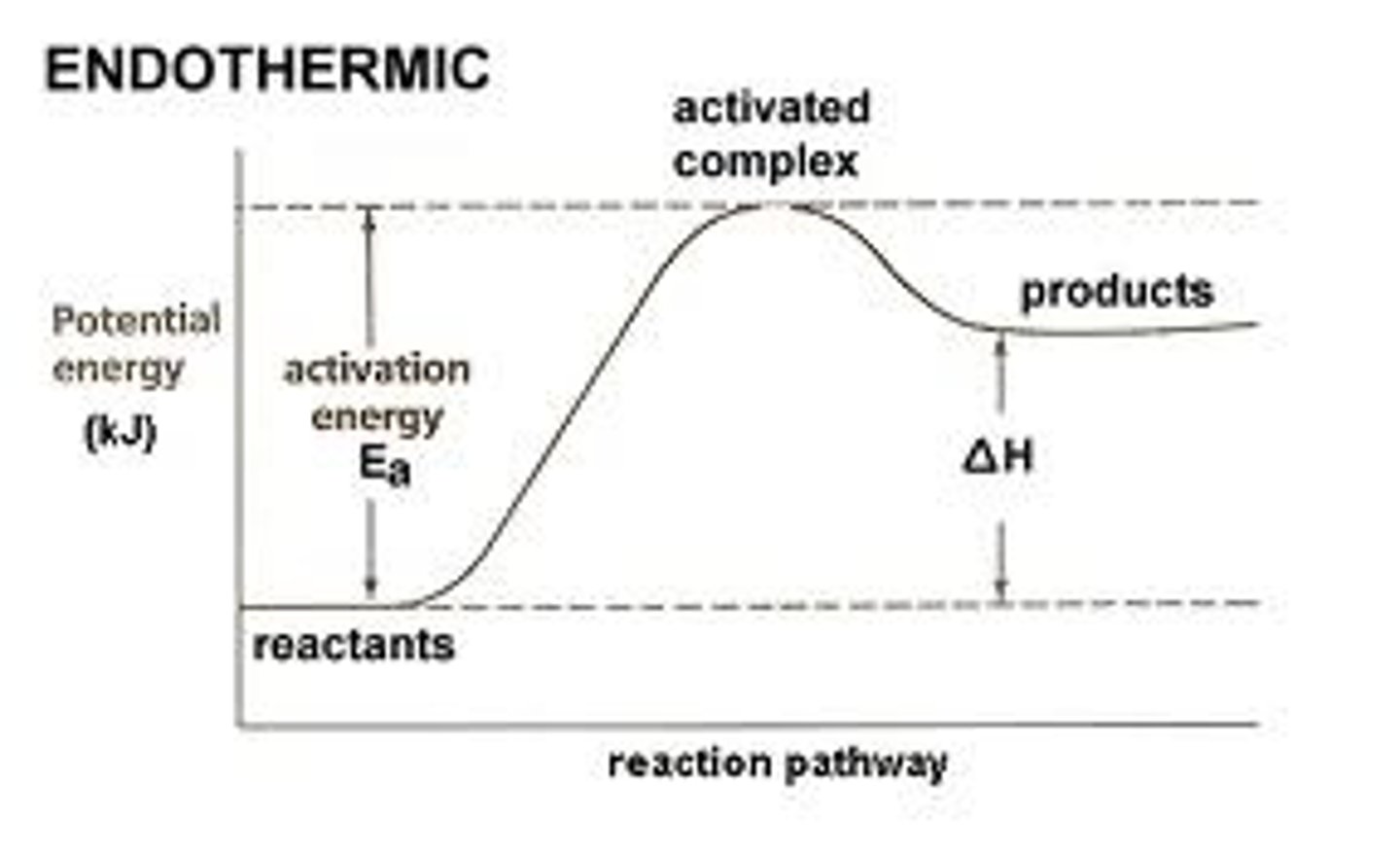
What happens when bonds are made?
Energy is released to the surroundings (exothermic). Therefore the temperature of the products is lower than the reactants.
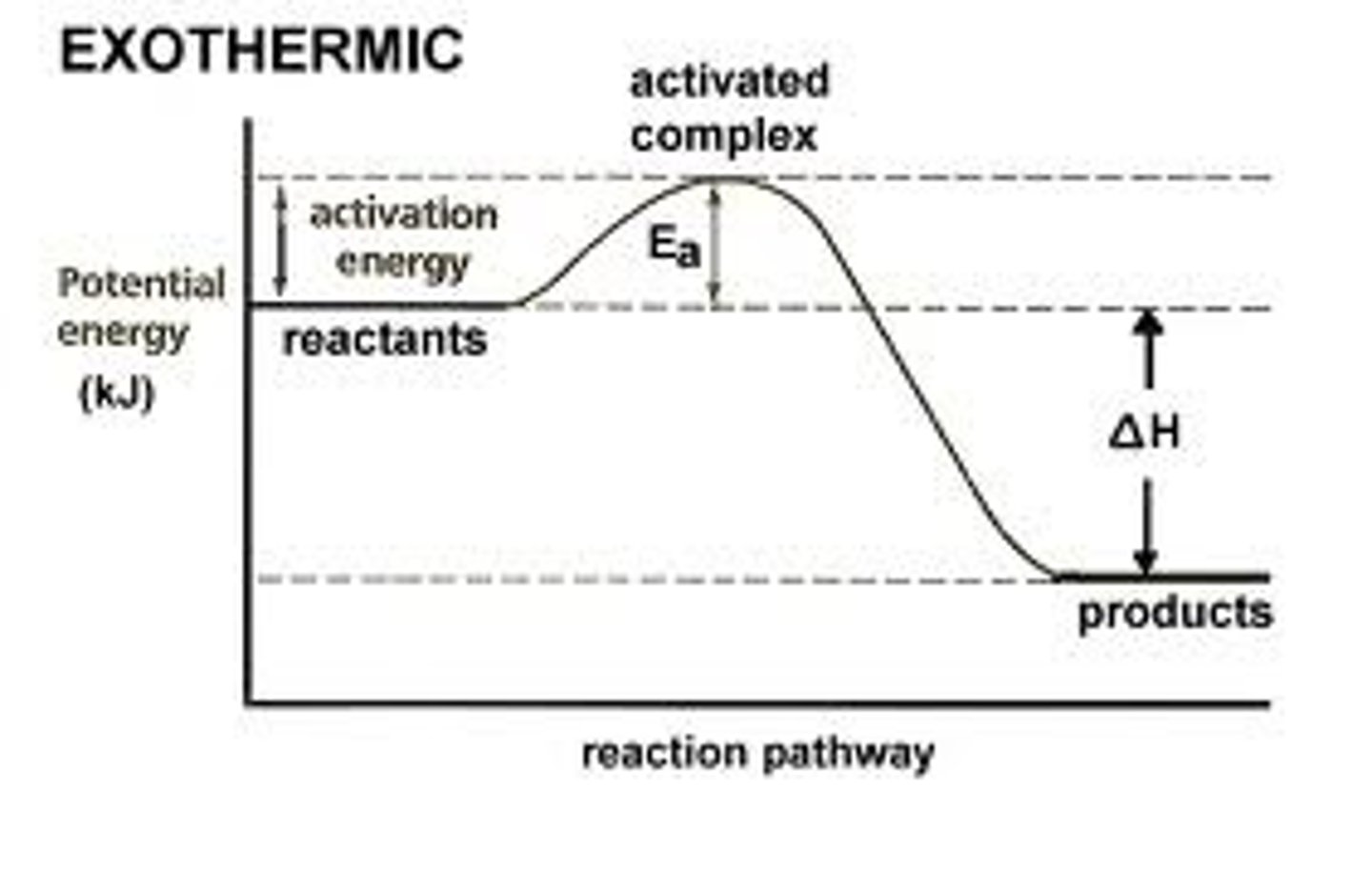
How can the rate of an enzyme-controlled reaction be measured?
There are 2 ways to measure the rate of an enzyme-controlled reaction:
1. The rate at which the substrate is used up e.g. rate at which starch is broken down by amylase
2. The rate at which the product is made e.g. rate at which lactase produces glucose.
What is an intracellular enzyme and examples of reactions catalysed by them?
An intracellular enzyme is one which occurs within a cell.
Examples of reactions catalysed by intracellular enzymes are: glycolysis and the Krebs cycle. Glycolysis takes place in the cytoplasm of the cell. The Krebs cycle takes place in the matrix of the mitochondria.
What is an extracellular enzyme and examples of reactions catalysed by them?
An extracellular enzyme occurs outside a cell.
Examples of reactions catalysed by extracellular enzymes are: chemical digestion within the gut/digestive system.
Why is heat generation an inevitable consequence of metabolic reactions?
The energy transfer in metabolic reactions is not 100% efficient and there is a great variation in the efficiency of the different metabolic reactions, therefore heat generation (energy transfer via heat) is an inevitable consequence of metabolic reactions.
Why is heat generation important to endotherms (warm-blooded animals)?
It maintains their constant internal body temperature so without the release of heat from these inefficient reactions, these organisms could not survive the low temperature extremes they can currently tolerate.
What are the 2 different types of metabolic pathways?
- Linear
- Cyclical
What is a linear metabolic pathway and one example?
A series of metabolic reactions that begin with one substance and end with another.
An example is glycolysis as part of respiration. Glycolysis begins with one 6-carbon compound and ends with the final products being 2 3-carbon compounds.
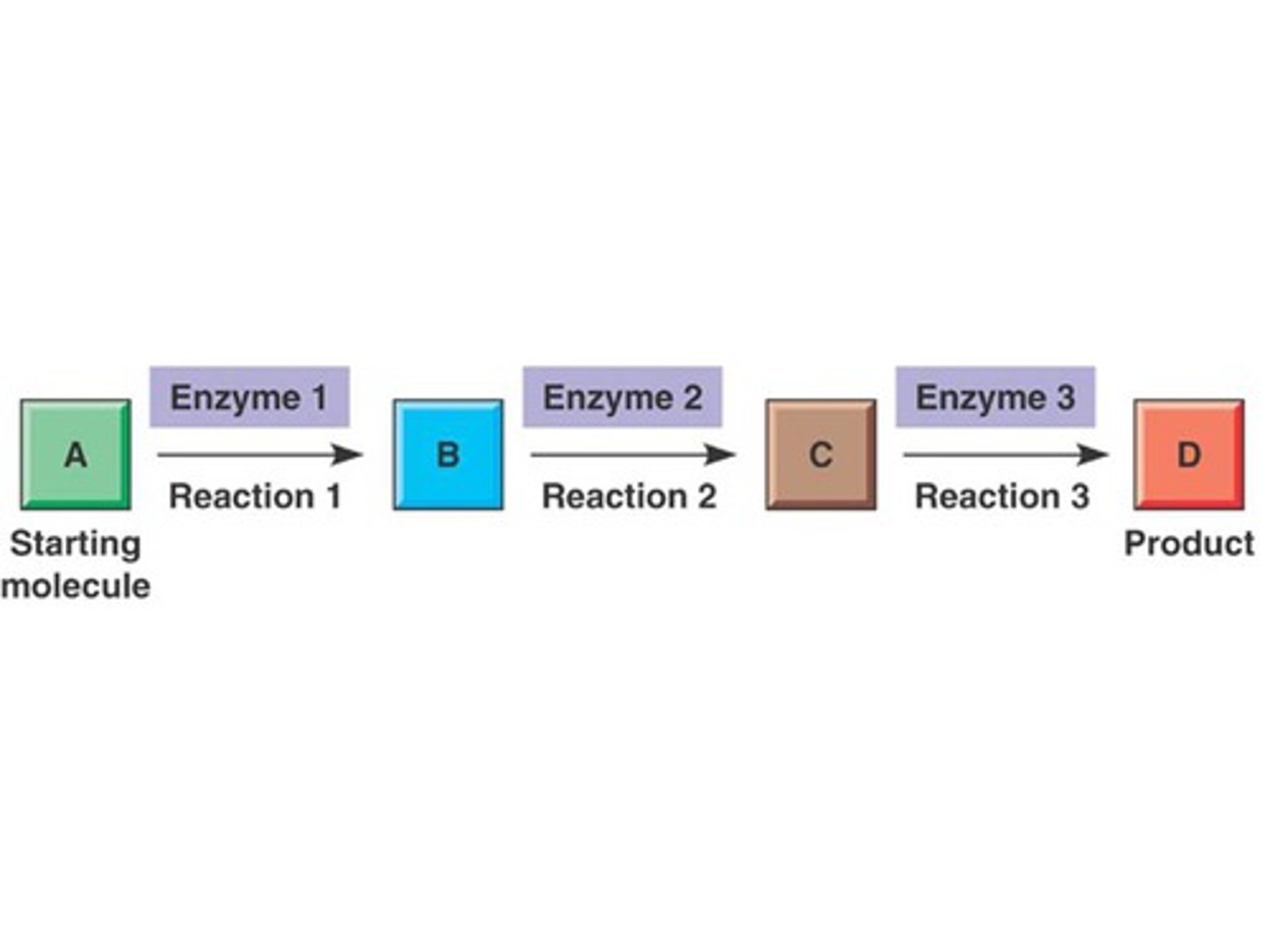
What is a cyclical metabolic pathway and two examples?
A series of metabolic reactions which begin and end with the same substance.
An example is the Krebs cycle as part of respiration. It begins and ends with the same 4-carbon compound. Another example in photosynthesis is the Calvin cycle which begins and ends with the same 5-carbon compound.
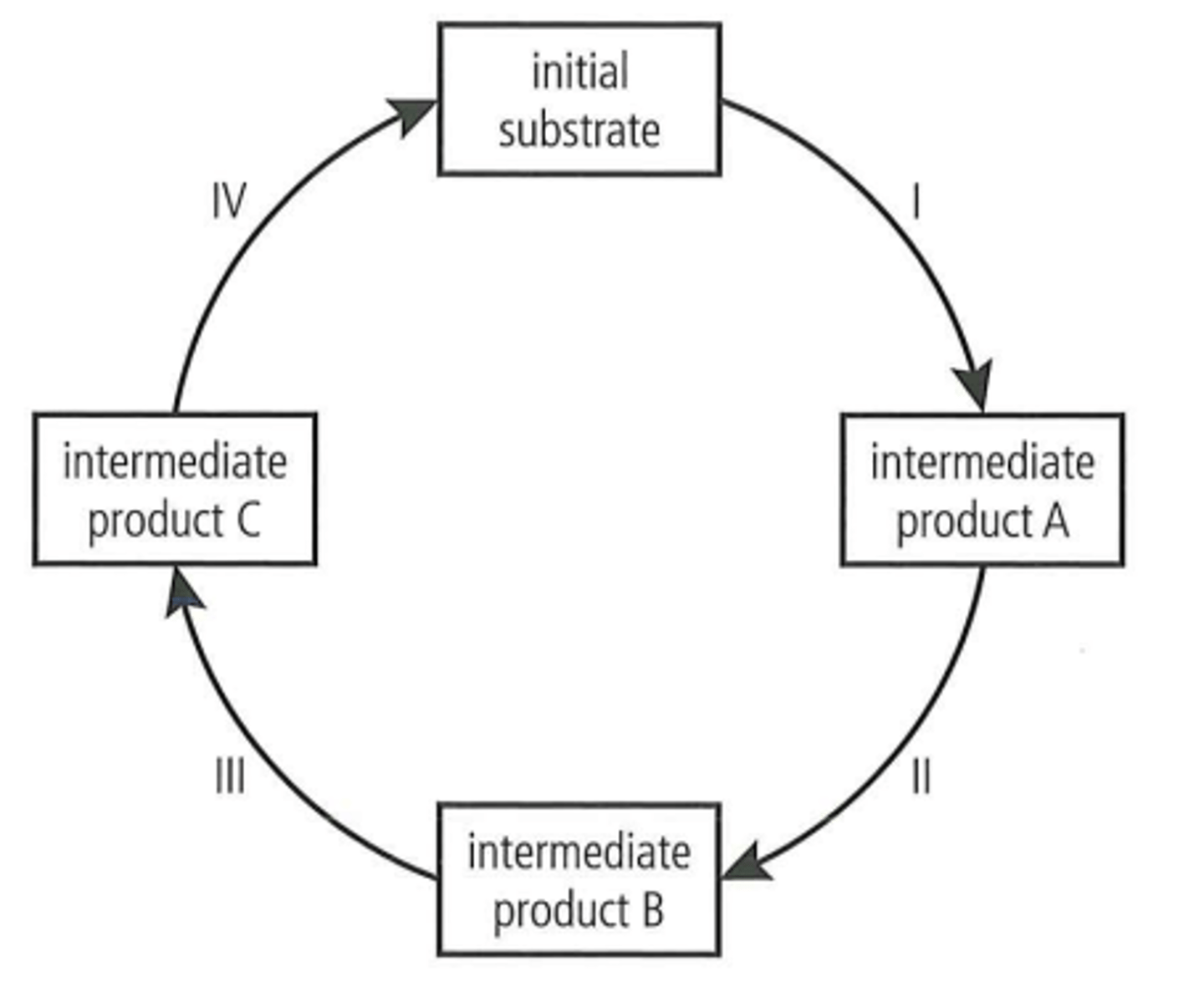
What is an inhibitor?
A molecule which decreases enzyme activity, slowing down the rate of reaction, by binding to it and altering/occupying the active site.
What are non-competitive inhibitors?
Non-competitive inhibitors hinder the activity of an enzyme by binding to the allosteric site and thus altering the tertiary structure of the protein and its active site so the substrate can no longer bind to it and form enzyme-substrate complexes as it ceases to be complementary. This binding is reversible.
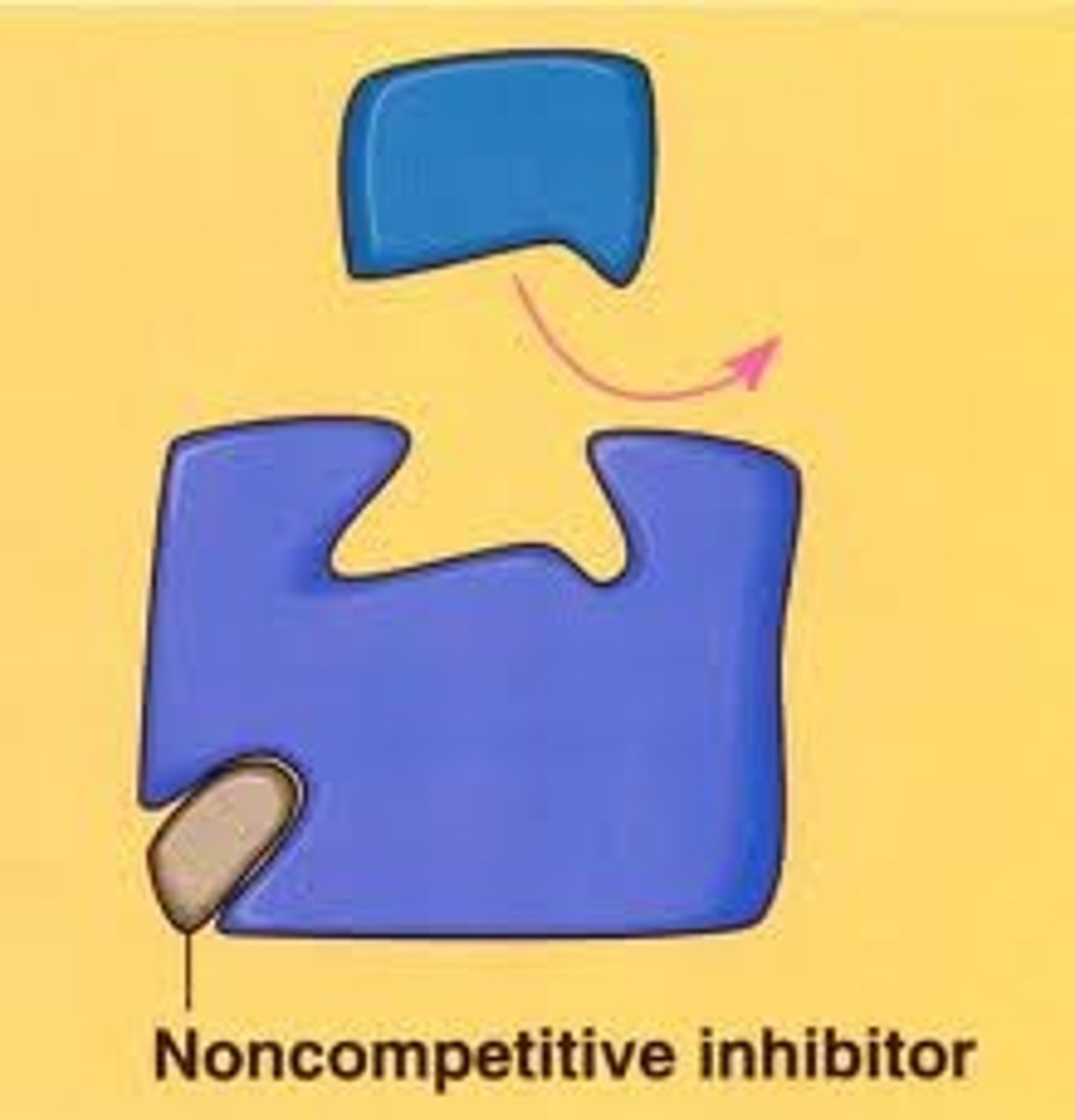
What is an example of a non-competitive inhibitor?
Metallic ions such as mercury bind to the sulphur groups of the amino acids in the enzyme which results in a change in the shape of the enzyme, inhibiting it.
What factors affect non-competitive inhibition and how?
- Concentration of substrate: by increasing the substrate concentration, this will increase the rate of reaction to an extent but eventually an increase will make no difference since the inhibitor binds to the allosteric rather than active site.
- Concentration of enzyme: by increasing the substrate concentration, this increases the number of enzymes available to bind to the substrate and form enzyme-substrate complexes with it.
Therefore, how is the effect of non-competitive inhibition overcome?
By increasing the enzyme concentration.
What are competitive inhibitors?
Competitive inhibitors hinder the activity of an enzyme by competing directly with the usual substrate for the active site, preventing the substrate from binding to it and forming an enzyme-substrate complex. Part of the inhibitor is complementary to the enzyme. This binding is reversible.

What is an example of a competitive inhibitor?
Statins:
People with high blood cholesterol can develop Coronary heart disease. When people have these high levels of cholesterol, doctors will prescribe a group of drugs known as statins. Statins act as competitive inhibitors as they combine with the active site of enzymes which catalyse the synthesis of cholesterol. Thus, they cause a reduction in the production of cholesterol therefore lowering the risk of cardiovascular disease.
What factors affect competitive inhibition and how?
- Concentration of substrate molecules: when there is a considerably higher concentration of substrate molecules, the substrate will outcompete the inhibitor. This doesn't particularly affect the rate of the reaction.
- Concentration of competitive inhibitor: when there is a considerably higher concentration of competitive inhibitor molecules, the inhibitor will outcompete the substrate for the active site of the enzyme and slow the rate of reaction.
Therefore, how is the effect of competitive inhibition overcome?
By increasing the concentration of the substrate.
What is feedback/end-product inhibition?
This is a process where a high concentration of a product of a metabolic pathway acts as an inhibitor (usually of the first enzyme) in the reaction that was making it. This is achieved by the end-product binding with the allosteric site of the first enzyme, thus bringing about inhibition. As the existing end-product is used up by the cell, the first enzyme is reactivated.
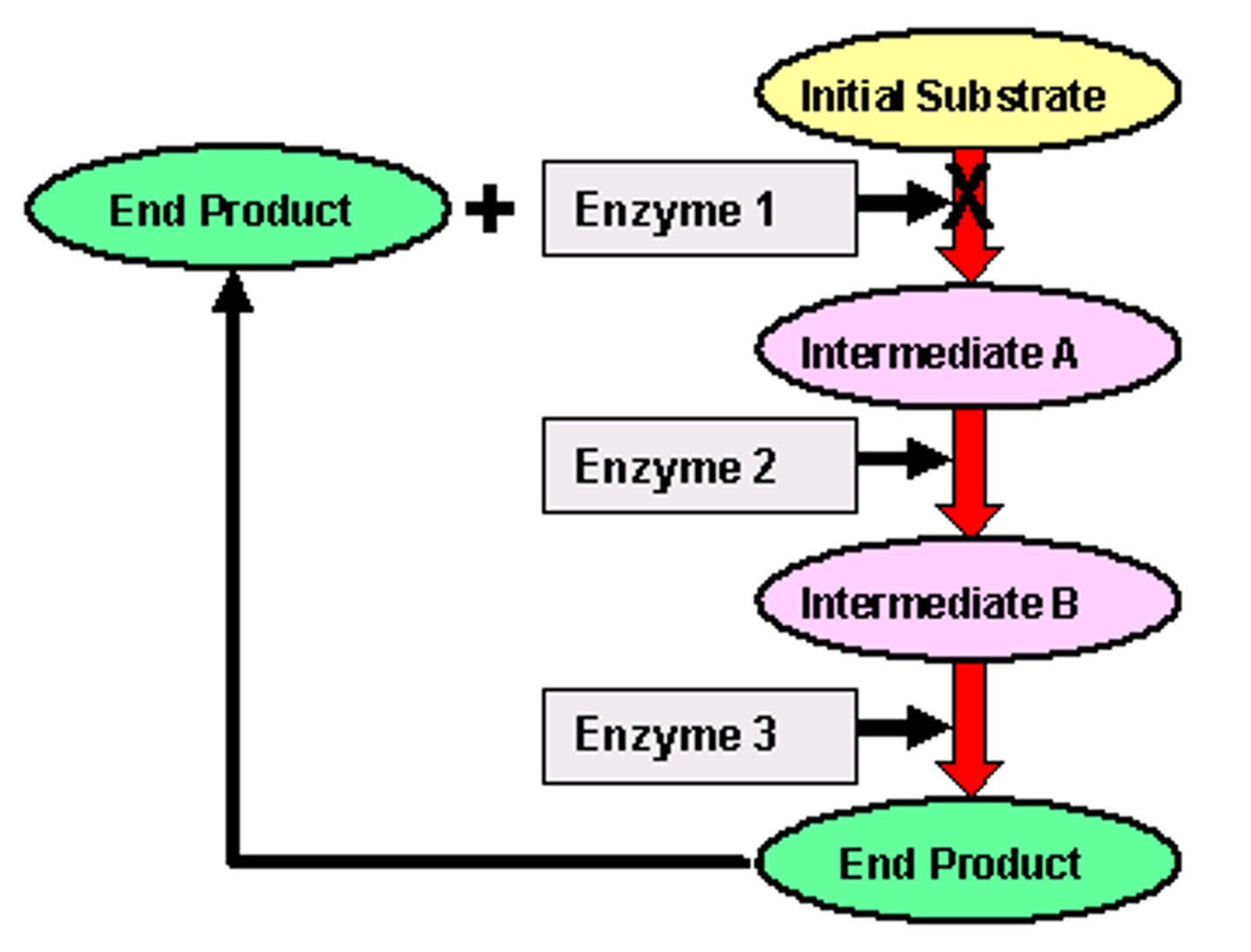
What are the advantages of end-product inhibition?
It can be used to regulate the chemical reactions in the cell to save energy and materials.
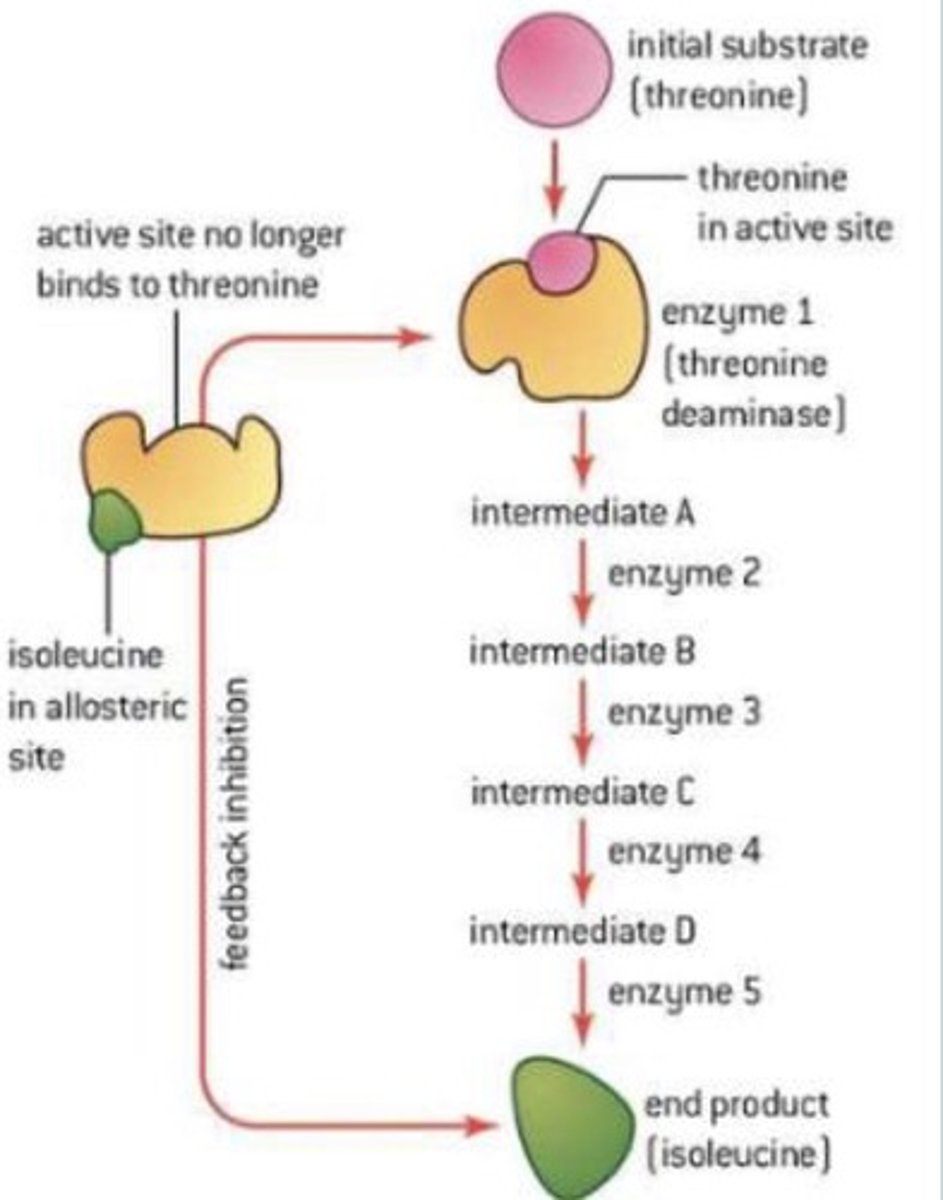
What is an example of end-product inhibition?
The synthesis of isoleucine in plants and bacteria:
1. Threonine combines with the enzyme threonine deaminase.
2. It goes through several intermediate conversions before producing isoleucine as its product.
3. The isoleucine combines with the allosteric site of the threonine deaminase, altering the active site so it can no longer combine with threonine.
4. This renders the path inactive and no more isoleucine is produced.
5. When isoleucine is used up the path reactivates.
What is mechanism-based inhibition?
The irreversible binding of an inhibitor with the active site of an enzyme, permanently changing its tertiary structure so the substrate cannot bind to it.
What is an example of mechanism-based inhibition?
Penicillin:
Penicillin irreversibly binds to the active site of an enzyme called transpeptidase that catalyses the last step in the formation of bacterial cell walls, inhibiting it. The defective cell wall prevents bacterial reproduction and causes the death of bacterial cells.
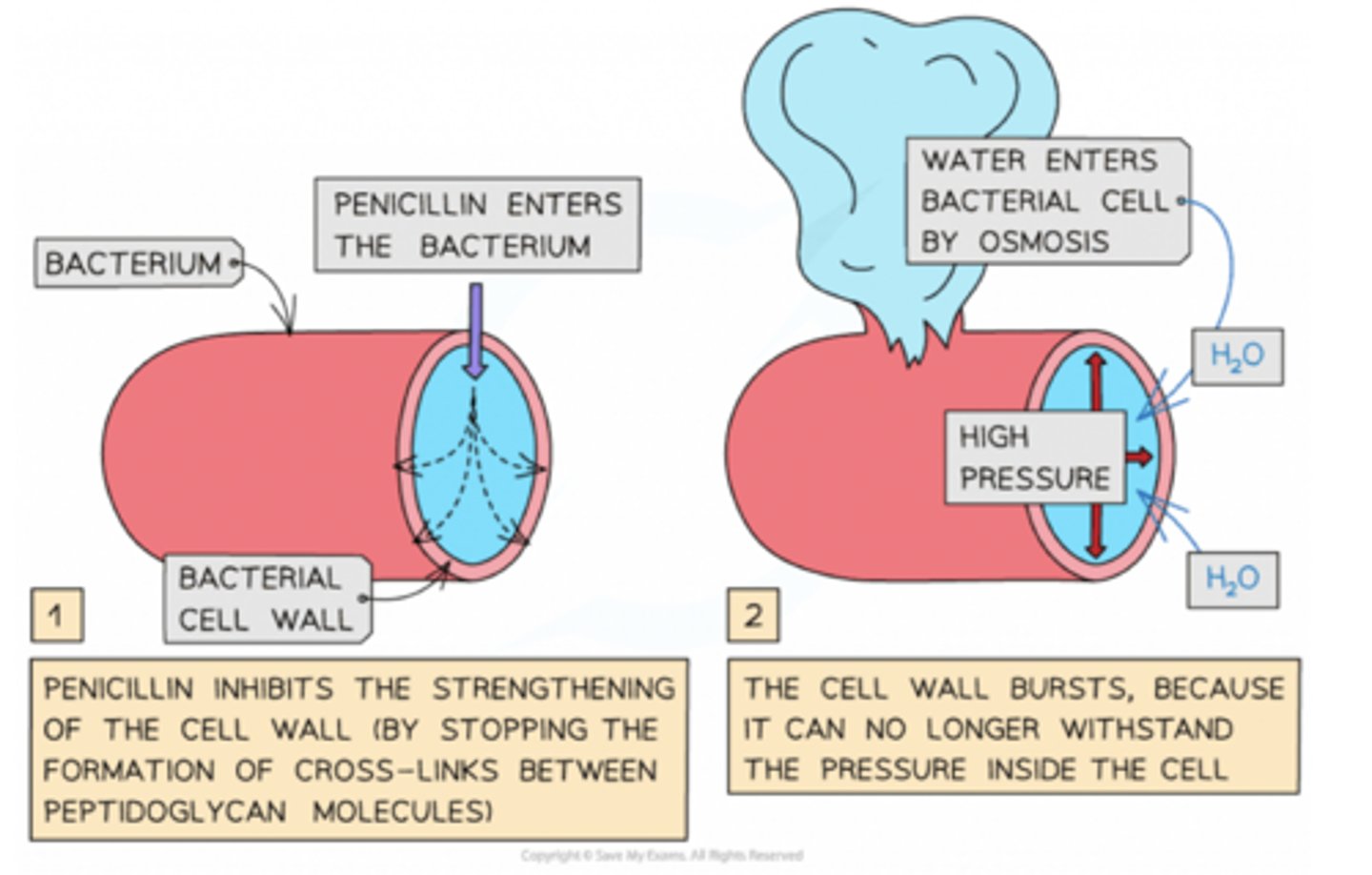
How can resistance to penicillin arise in bacteria?
- Certain strains of bacteria have developed a mutation that allows them to produce an enzyme called penicillinase. This enzyme breaks down penicillin.
- Certain strains of bacteria have developed mutations in the transpeptidase active site so that penicillin can no longer bind it, resulting in bacterial resistance.