CHEM4 - Last minute revision moment
5.0(1)
Card Sorting
1/46
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
47 Terms
1
New cards
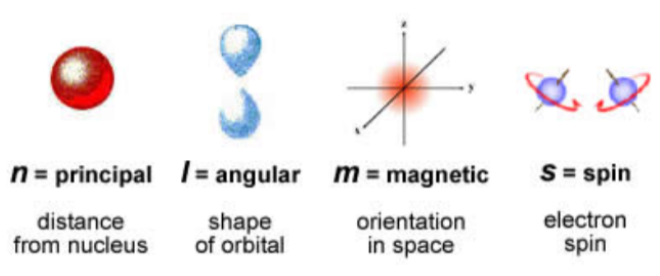
List the 4 quantum numbers and what they tell us about an electron orbital
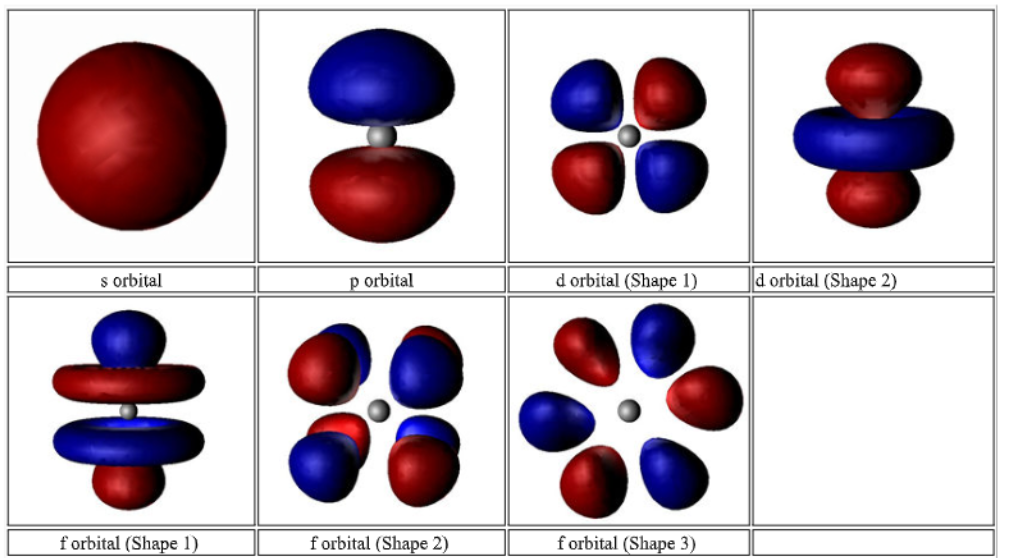
* n - Principal (distance from nucleus/ energy level)
* l - Angular (Shape of orbital s,p,d,f)
* M - Magnetic (Orientation in space/ number of orbitals available_)
* s - spin Which direction each electron can spin
* l - Angular (Shape of orbital s,p,d,f)
* M - Magnetic (Orientation in space/ number of orbitals available_)
* s - spin Which direction each electron can spin
2
New cards
Pauli Exclusion Principle:
\
\
No two electrons in an atom have the same set of 4 quantum numbers (since quantum numbers can only take on two values it can only be +1/2 or -1/2 i.e opposite spin)
3
New cards
Hunds rule
Electrons will enter the lowest available energy level first and then singly into orbitals of equal energy, when they are available before pairing up (aka will try to only fill 1 electron in each orbital before adding in more)
4
New cards
Afbau principle
In the ground state of an atom the electrons fill into orbital in order from lowest energy to highest.
5
New cards
Degenerate electrons
Electrons that are in the same energy level
6
New cards
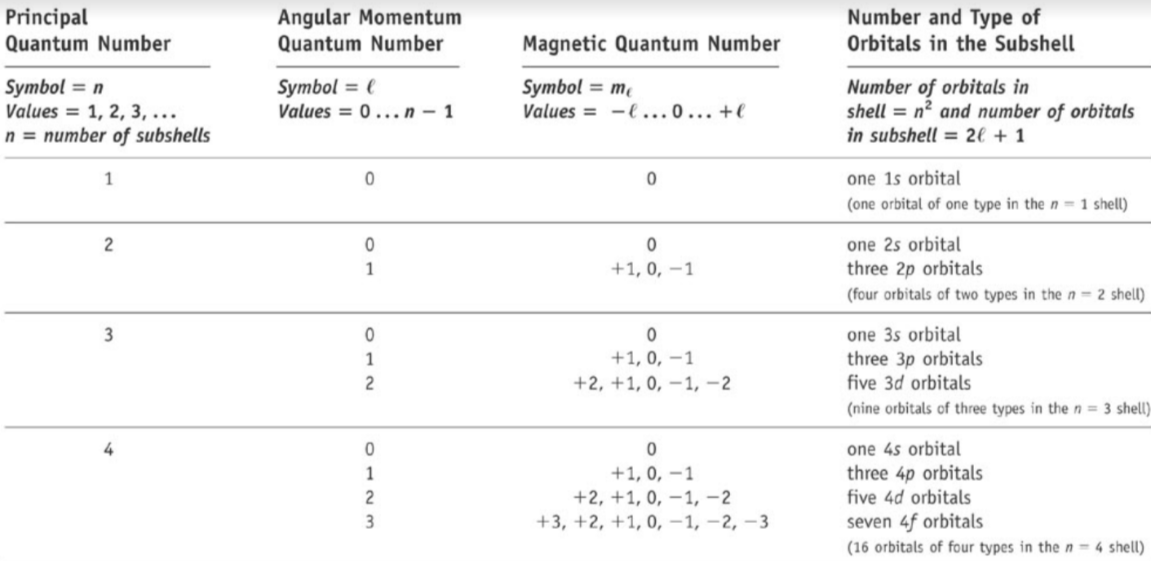
1\. Principal (shell) quantum number - n
* Looks at main energy levels
* Tip: total number of electrons in an energy level is 2n^2
* Can take on whole values: 1,2,3…
* Tip: total number of electrons in an energy level is 2n^2
* Can take on whole values: 1,2,3…
7
New cards
2\. Angular momentum (subshell) quantum number - l
* Describes the sublevel in n
* Each energy level has n sublevels
* l can either be 0 or n-1
* Each energy level has n sublevels
* l can either be 0 or n-1
8
New cards
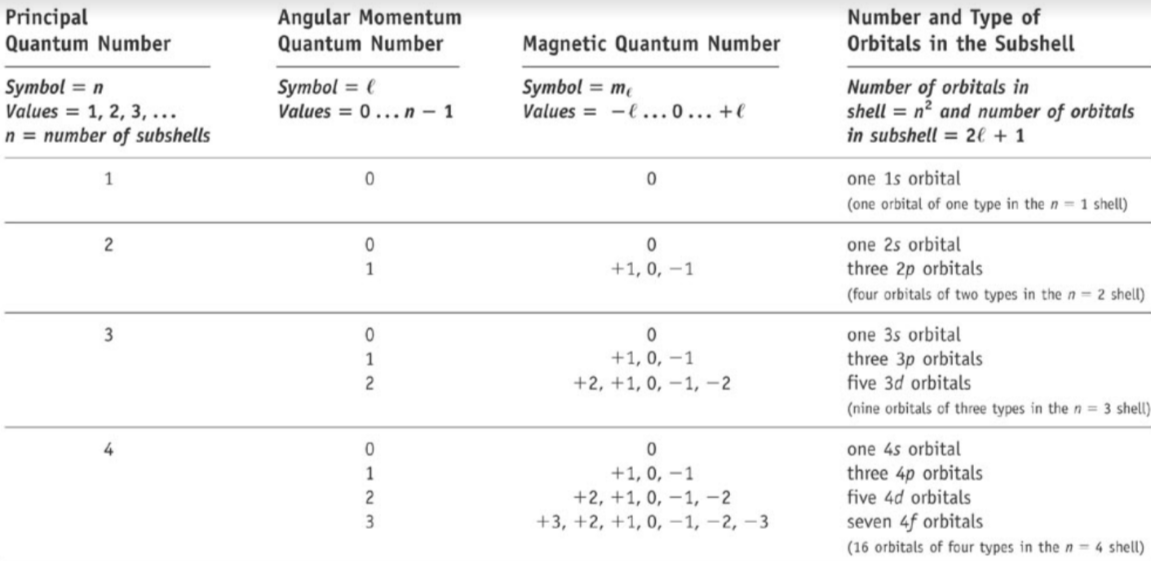
3\. Magnetic quantum number - ml
* Describes the number and orientation of orbitals within l
* Values = -l < ml < +l
* Values = -l < ml < +l
9
New cards
4\. Spin quantum number - ms
Direction of electron spin about its own spin.
* Can either be clock-wise or counter-clockwise
* Values of Ms care either: +1/2 or -1/2
* Can either be clock-wise or counter-clockwise
* Values of Ms care either: +1/2 or -1/2
10
New cards
Electron configuration
Representing how electrons have filled each orbital for an element
* Full - write out all the electrons which inhabit each orbital
* Partial - Write noble gas of row before to indicate previously filled orbital
* Full - write out all the electrons which inhabit each orbital
* Partial - Write noble gas of row before to indicate previously filled orbital
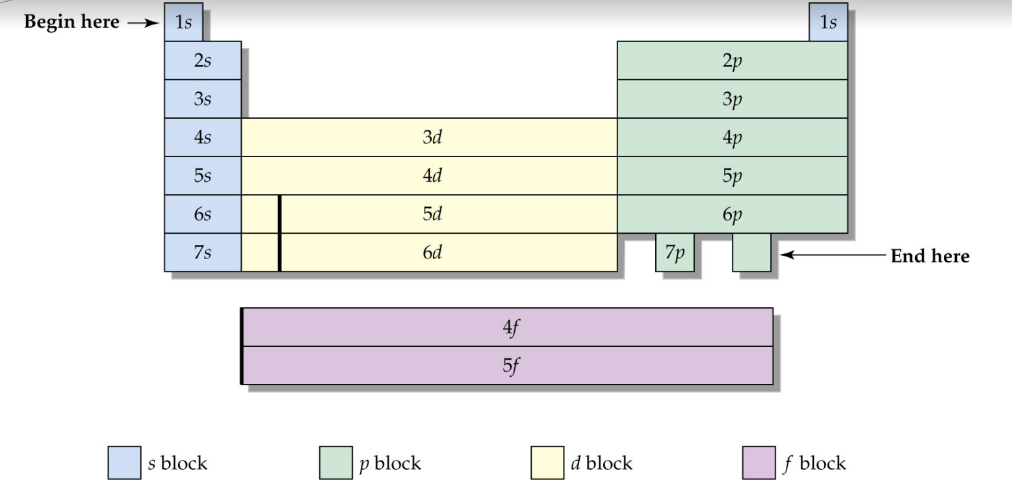
11
New cards
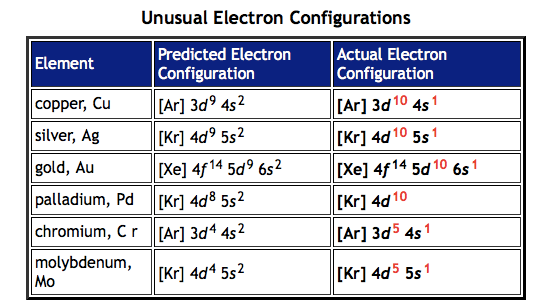
Exceptions to electron configuration
Main ones are copper and chromium but Molybdenum, Silver and gold apply
12
New cards
Isoelectronic series + trend
A group of atoms/ ions with the same electron configuration
* As the isoelectronic series goes across, the radius decreases since theres more protons and thus a stronger electrostatic force
* As the isoelectronic series goes across, the radius decreases since theres more protons and thus a stronger electrostatic force
13
New cards
Electron configurations for ions
When removing electrons from ions, make sure you’re removing from the shell with the *highest n value,* then prioritise energy. Although technically 4d has more energy than 5s, 5s is still further out so those electrons will get yoinked
14
New cards
Electrostatic attraction
When negatively charged atom is attracted towards positively charged atom and vice-versa
15
New cards
Atomic radius
half of the distance between the 2 nuclei of atoms when the atoms are joined
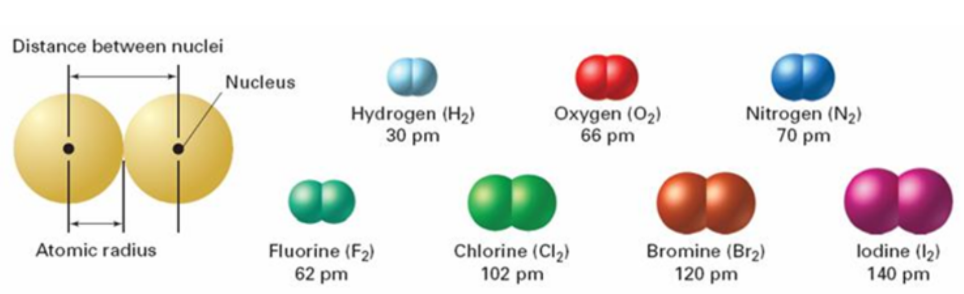
16
New cards
Atomic radii - Trend
* **Decreases across a period** as the electrons are relatively the same distance from the nucleus (in the same n energy level) but the number of protons in the nucleus is increasing for each consecutive member in a period, so the nucleus has a greater attraction for these valence electrons and pulls them in closer to the nucleus.
* **Increases down a group** as for each consecutive member there is an additional n electron energy level, which both increases the volume of the atom and increases the shielding of the nucleus from the valence electrons so decreases the attraction of the nucleus for these electrons.
* **Increases down a group** as for each consecutive member there is an additional n electron energy level, which both increases the volume of the atom and increases the shielding of the nucleus from the valence electrons so decreases the attraction of the nucleus for these electrons.
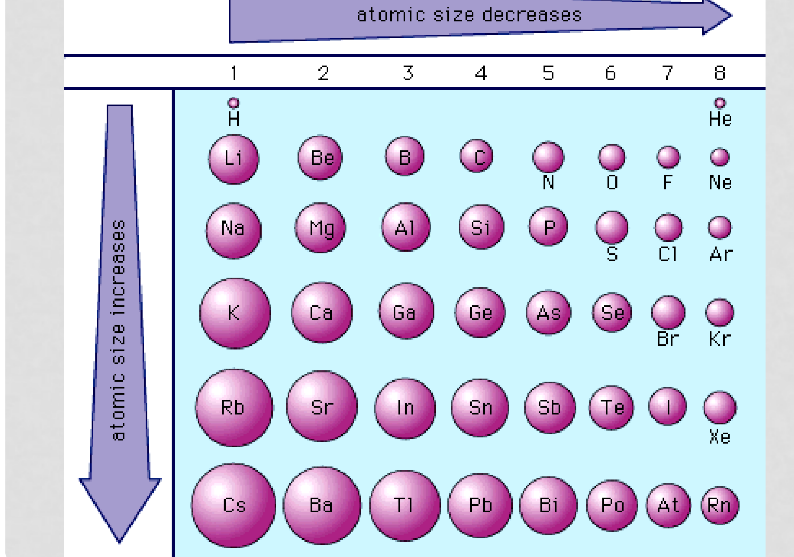
17
New cards
Ionic radii
* If an atom loses electrons the radius of the ==cation is smaller== than the parent atom, as electrons have been lost from the outermost energy level. The remaining electrons are strongly attracted to the nucleus, as the number of protons in the nucleus is now greater than the electrons around the ion.
* If an atom gains electrons to form an **anion**, the ==radius is larger== than the parent atom. This is due to increased repulsion between the increased number of electrons and a lesser attraction of the protons for the increased number of electron.
* If an atom gains electrons to form an **anion**, the ==radius is larger== than the parent atom. This is due to increased repulsion between the increased number of electrons and a lesser attraction of the protons for the increased number of electron.
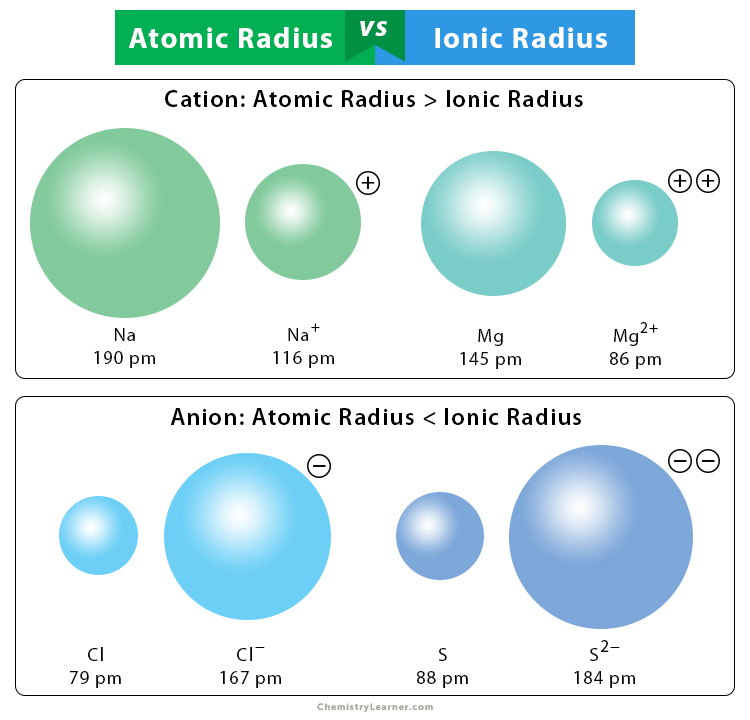
18
New cards
Ionisation Energy (IE)
The energy required to remove one mole of electrons from one mole of gaseous atoms.
* **In general** Ionisation energy increases across a period but there are exceptions
* **IE decreases down a group:** Additional n level, increases distance of electron from nucleus → increased shielding → Less attraction
* **In general** Ionisation energy increases across a period but there are exceptions
* **IE decreases down a group:** Additional n level, increases distance of electron from nucleus → increased shielding → Less attraction

19
New cards
Factors affecting ionisation energy
* Charge: More protons → more positve charge → more electrostatic attraction
* Distance of electron from nucleus: Attraction decreases with distance
* Number of electrons between outer shell and nucleus: Pull lessened by inner electron (i.e shielding, screening)
* If electon is alone in orbital/ paired: Electron in same orbital repel each other → offsets attraction → easily removed
* Distance of electron from nucleus: Attraction decreases with distance
* Number of electrons between outer shell and nucleus: Pull lessened by inner electron (i.e shielding, screening)
* If electon is alone in orbital/ paired: Electron in same orbital repel each other → offsets attraction → easily removed
20
New cards
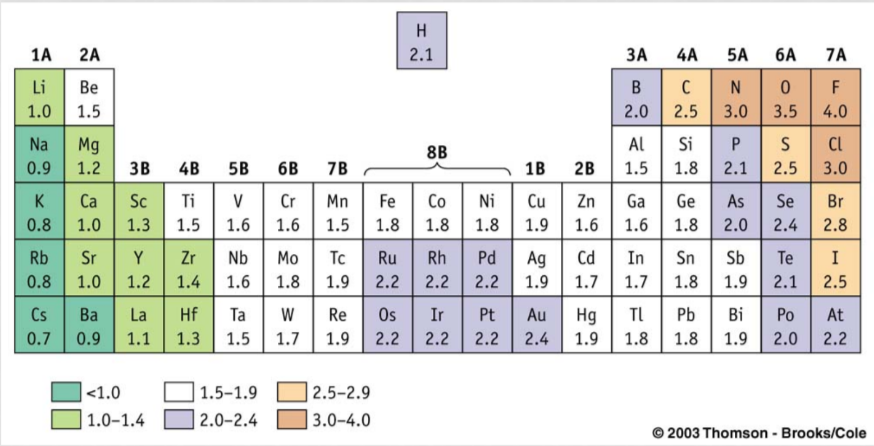
Electronegativity
Electronegativity is a measure of the tendency of a bonded atom to attract a bonding pair of electrons.
* I**ncreases across a period** with an increasing number of protons in the nucleus.
* **Decreases down a group** with increased shielding of the nucleus.
1\. If the electronegativity difference (usually called ΔEN) is less than 0.5, then the bond is nonpolar covalent.
2\. If the ΔEN is between 0.5 and 1.6, the bond is considered polar covalent
3\. If the ΔEN is greater than 2.0, then the bond is ionic.
4\. If the ΔEN is between 1.6 and 2.0 and if a metal is involved, then the bond is considered ionic. If only nonmetals are involved, the bond is considered polar covalent.
* I**ncreases across a period** with an increasing number of protons in the nucleus.
* **Decreases down a group** with increased shielding of the nucleus.
1\. If the electronegativity difference (usually called ΔEN) is less than 0.5, then the bond is nonpolar covalent.
2\. If the ΔEN is between 0.5 and 1.6, the bond is considered polar covalent
3\. If the ΔEN is greater than 2.0, then the bond is ionic.
4\. If the ΔEN is between 1.6 and 2.0 and if a metal is involved, then the bond is considered ionic. If only nonmetals are involved, the bond is considered polar covalent.
21
New cards
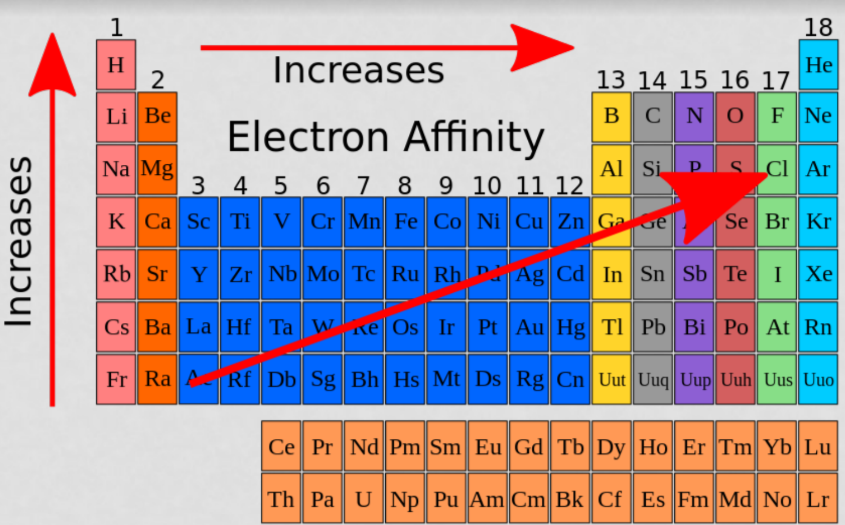
Electron affinity
Electron affinity is the energy required (or released) when an electron is added to a gaseous atom or ion.
* Generally **increases going up a group and increases left to right across a period**
The first electron affinity is the energy released when one mole of gaseous atoms each acquires an electron to form one mole of gaseous 1- ions.
* 1st one is usually exothermic because the atom has to slow down for the bond to form
2nd EA is endothermic because the electron is being forced into a small, very electron-dense space.(aka its sick of electrons and doesn’t feel like having another one lol)
* Generally **increases going up a group and increases left to right across a period**
The first electron affinity is the energy released when one mole of gaseous atoms each acquires an electron to form one mole of gaseous 1- ions.
* 1st one is usually exothermic because the atom has to slow down for the bond to form
2nd EA is endothermic because the electron is being forced into a small, very electron-dense space.(aka its sick of electrons and doesn’t feel like having another one lol)
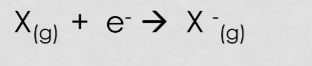
22
New cards
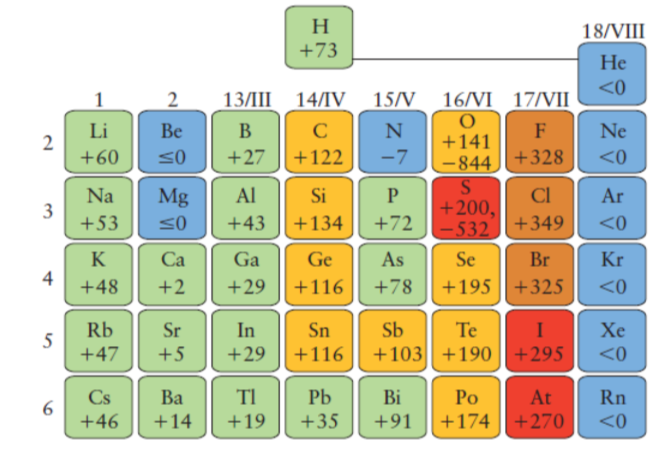
Factors affecting EA
* Nuclear charge and electron-electron repulsion (latter is significant here)
* One reason for this is that the electrons contained in the inner shells of the atom exert a collective negative charge that partially cancels the charge of the nucleus, thus exerting a so-called shielding effect which diminishes the tendency for negative ions to form. Because of these opposing effects, the periodic trends in electron affinities are not as clear as are those of ionization energies.
* In general, we can say that electron affinities become more exothermic as we move from left to right across a period (owing to increased nuclear charge and smaller atom size). There are some interesting irregularities, however:
* In the Group 2 elements, the filled 2s orbital apparently shields the nucleus so effectively that the electron affinities are slightly endothermic.
* The Group 15 elements have rather low values, due possibly to the need to place the added electron in a half-filled p orbital; why the electron affinity of nitrogen should be endothermic is not clear. The vertical trend is for electron affinity to become less exothermic in successive periods owing to better shielding of the nucleus by more inner shells and the greater size of the atom, but here also there are some apparent anomalies.
* One reason for this is that the electrons contained in the inner shells of the atom exert a collective negative charge that partially cancels the charge of the nucleus, thus exerting a so-called shielding effect which diminishes the tendency for negative ions to form. Because of these opposing effects, the periodic trends in electron affinities are not as clear as are those of ionization energies.
* In general, we can say that electron affinities become more exothermic as we move from left to right across a period (owing to increased nuclear charge and smaller atom size). There are some interesting irregularities, however:
* In the Group 2 elements, the filled 2s orbital apparently shields the nucleus so effectively that the electron affinities are slightly endothermic.
* The Group 15 elements have rather low values, due possibly to the need to place the added electron in a half-filled p orbital; why the electron affinity of nitrogen should be endothermic is not clear. The vertical trend is for electron affinity to become less exothermic in successive periods owing to better shielding of the nucleus by more inner shells and the greater size of the atom, but here also there are some apparent anomalies.
23
New cards
Ionic bonding + list factors affecting lattice
Bonding between anion and cation
**Lattice energy -** The enthalpy change when 1 mole of ions in a solid ionic lattice is formed (exothermic)
**Factors:**
* **Charge -** Stronger electrostatic attraction
* **Size -** Distance between nuclei affects attraction
ie small ions + large charges are ideal
**Lattice energy -** The enthalpy change when 1 mole of ions in a solid ionic lattice is formed (exothermic)
**Factors:**
* **Charge -** Stronger electrostatic attraction
* **Size -** Distance between nuclei affects attraction
ie small ions + large charges are ideal
24
New cards
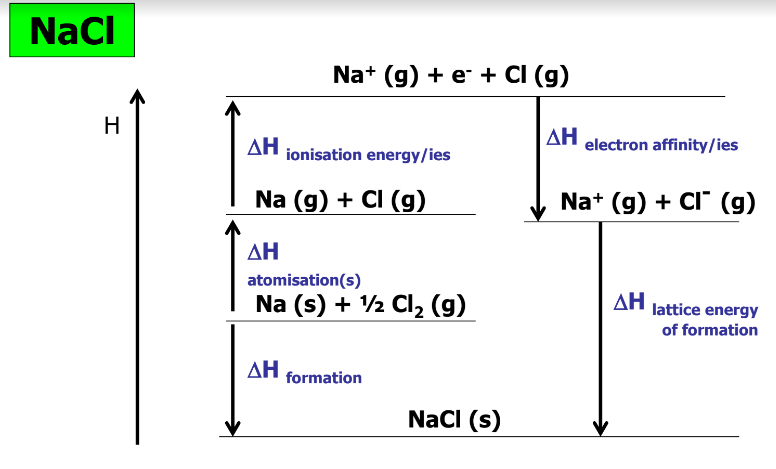
Born Haber Cycles - List all enthalpies involved
∆Hformation = sum of all other ∆H’s
**Lattice energy -** The enthalpy change when 1 mole of ions in a solid ionic lattice is formed (exothermic)
**Enthalpy of formation** - Enthalpy change when 1 mol of a lattice is formed from elements under standard conditions (exo)
**Enthalpy of atomisation -** Energy required to change 1 mol of an atom into a 1 mol of its gaseous version
**IE -** E required to remove 1 mol of electrons from 1 mol of gaseous metal atom
**EA -** E required to add 1 mol of electrons to 1 mol of gaseous atoms to form a charged ion
**Lattice energy -** The enthalpy change when 1 mole of ions in a solid ionic lattice is formed (exothermic)
**Enthalpy of formation** - Enthalpy change when 1 mol of a lattice is formed from elements under standard conditions (exo)
**Enthalpy of atomisation -** Energy required to change 1 mol of an atom into a 1 mol of its gaseous version
**IE -** E required to remove 1 mol of electrons from 1 mol of gaseous metal atom
**EA -** E required to add 1 mol of electrons to 1 mol of gaseous atoms to form a charged ion
25
New cards
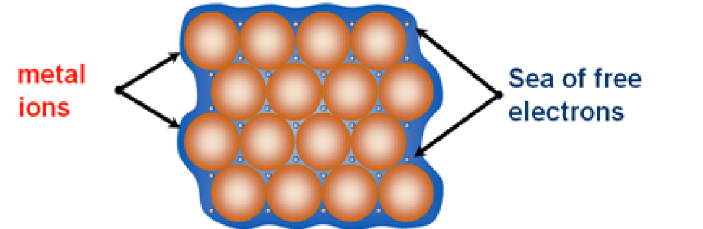
Metallic bonding + factors affecting it
* Atoms are tightly packed together in a giant lattice
* Outer electrons of valence shells delocalise, creating a **sea of electrons**
* Attraction is known as **metallic bonding**
\
Factors affecting it include charge and size → bigger charge → more attraction
* Outer electrons of valence shells delocalise, creating a **sea of electrons**
* Attraction is known as **metallic bonding**
\
Factors affecting it include charge and size → bigger charge → more attraction
26
New cards
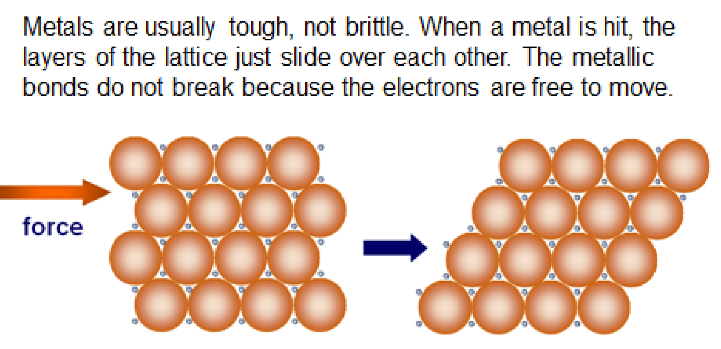
Properties of metallic lattices
* Melting points decrease down a group
* Good conductors of heat and electricity
Metals are malleable (not brittle) and ductile (can be beaten into wires)
* Good conductors of heat and electricity
Metals are malleable (not brittle) and ductile (can be beaten into wires)
27
New cards
VSEPR shapes - Linear
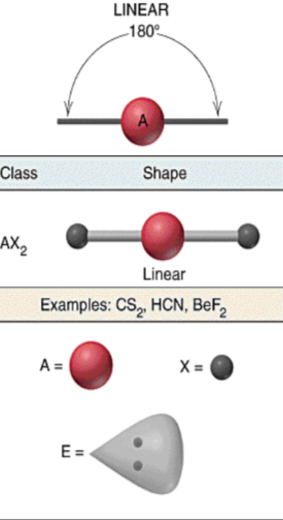
28
New cards
VSEPR shapes - Trigonal planar
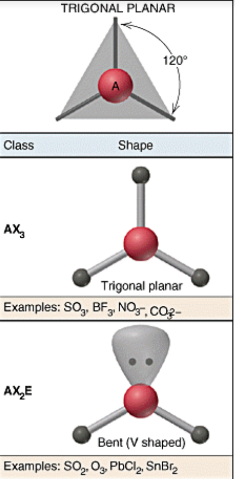
29
New cards
VSEPR shapes - tetrahedral
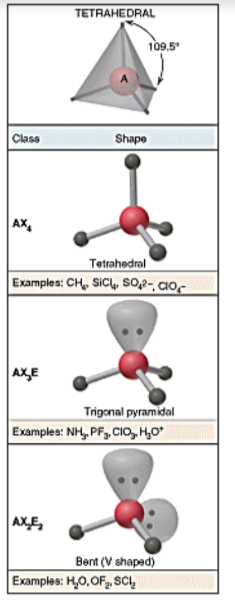
30
New cards
VSEPR Shapes - Trigonal bipyramidal
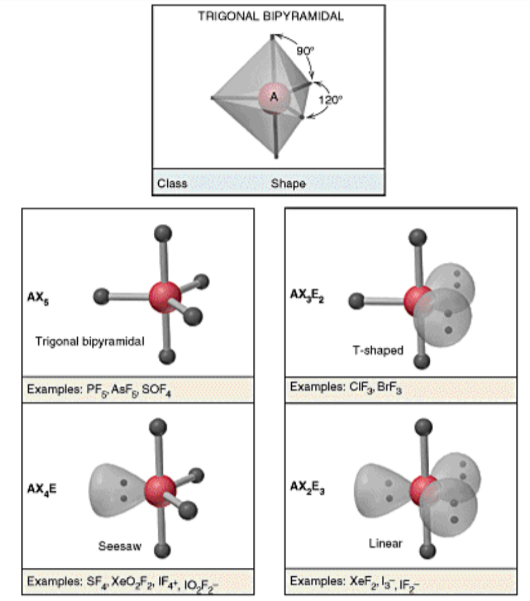
31
New cards
VSEPR shapes - octahedral
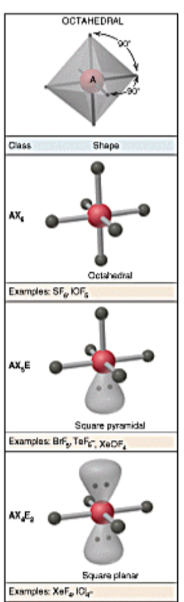
32
New cards
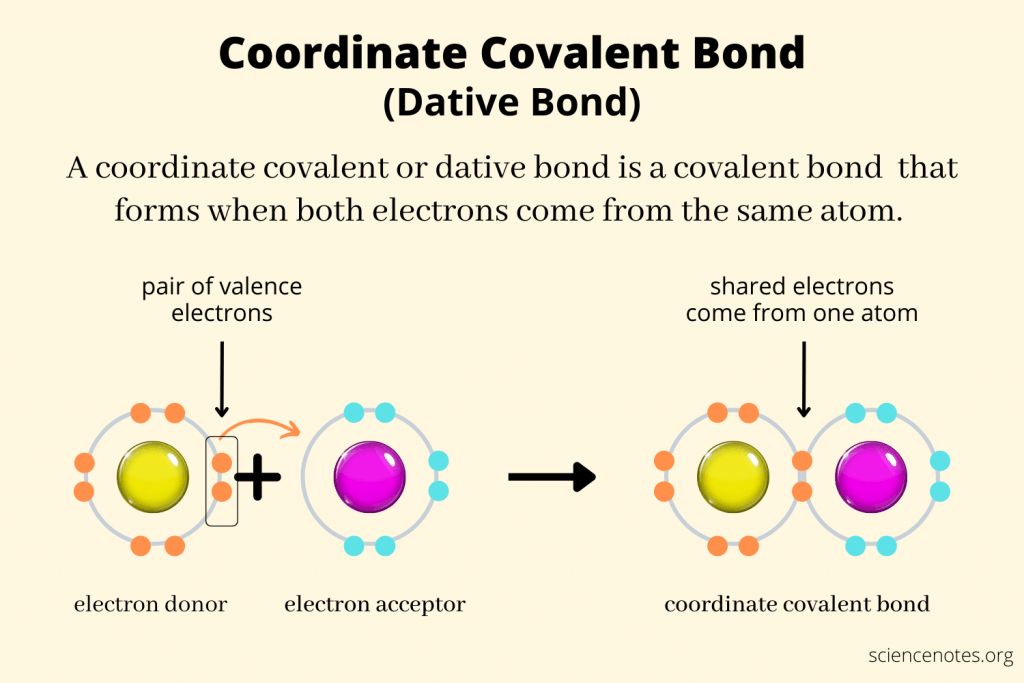
Dative/ Coordinate bonds
is a covalent bond (a shared pair of electrons) in which both electrons come from the same atom.
Note: Represented as an arrow
Note: Represented as an arrow
33
New cards
Formal charges
Charge of each atom in a lewis structure
Calculated by: VE - (# of bonds + # of dots)
* Molecule will gravitate towards a compound which has the lowest formal charges
Calculated by: VE - (# of bonds + # of dots)
* Molecule will gravitate towards a compound which has the lowest formal charges
34
New cards
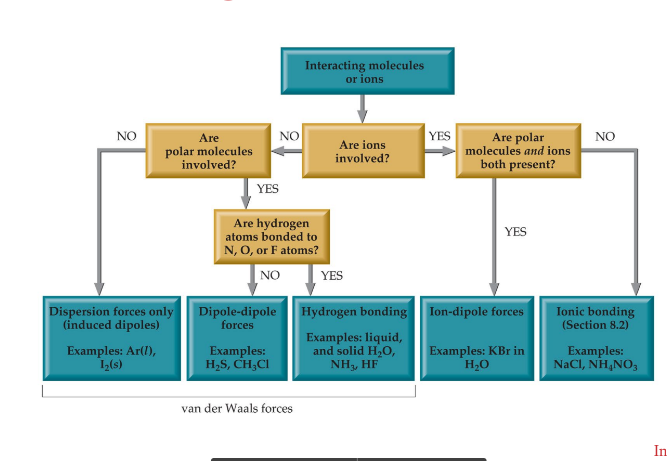
Intermolecular forces
* Hydrogen bonding
* Dipole-dipole interactions
* Ion-Dipole Interactions
* London dispersion forces
* Dipole-dipole interactions
* Ion-Dipole Interactions
* London dispersion forces
35
New cards
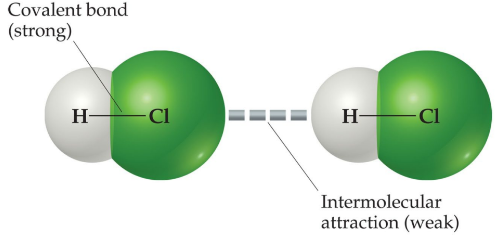
Intermolecular forces vs intramolecular forces
The attractions between molecules are not nearly as strong as the intramolecular attractions that hold compounds together. (Ionic, covalent and metallic bonds.)
They are, however, strong enough to control physical properties such as boiling and melting points, vapor pressures, and viscosities.
They are, however, strong enough to control physical properties such as boiling and melting points, vapor pressures, and viscosities.
36
New cards
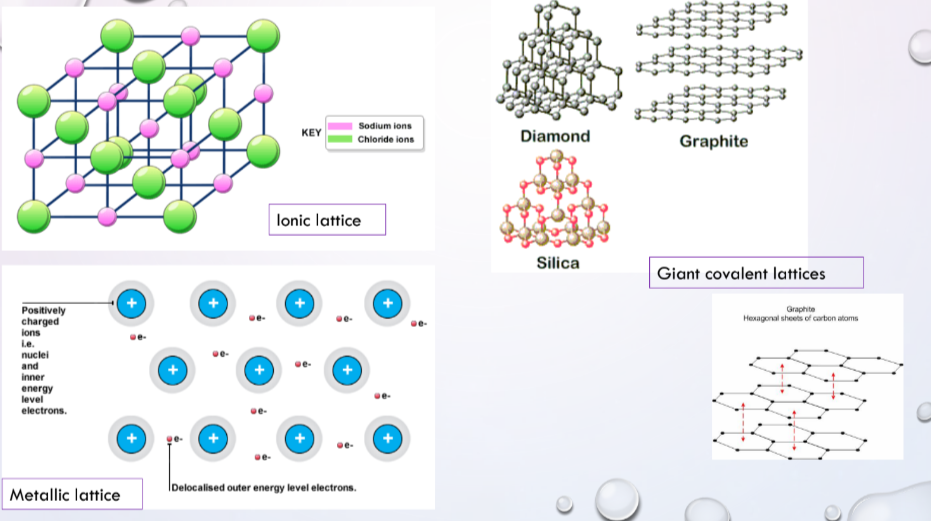
List structures / chemical structures
* **Ionic lattices**
* three dimensional arrangement of positive and negative ions, electrostatic attraction of the opposite charges
* no *mobile* electrons, evidenced by no conductivity of electricity in solid structure
* attraction of ions at lattice points to each other is strong, evidenced by high melting point of solid
* **Metallic** **Lattices**
* attraction of the positive ions for the valence electrons is strong, evidenced by high melting point of solids
* **Giant covalent lattices**
* covalent bonds between atoms are very strong, difficult to overcome, evidenced by very high melting point of solids
* all electrons are localised in bonds and are not free to move away, evidenced by no electrical conductivity in the solid
* **Covalent molecules**
* if a solid forms then the molecules are at the lattice points with intermolecular forces of attraction between the molecules
* on melting it is not the covalent bonds inside the molecule that are broken but the attractive forces between the molecules which are overcome, evidenced by relatively low melting points of solids
* no free electrons that can move off the molecule, they are localised in bonds, evidenced by solids of covalent molecule material not conducting electricity
* three dimensional arrangement of positive and negative ions, electrostatic attraction of the opposite charges
* no *mobile* electrons, evidenced by no conductivity of electricity in solid structure
* attraction of ions at lattice points to each other is strong, evidenced by high melting point of solid
* **Metallic** **Lattices**
* attraction of the positive ions for the valence electrons is strong, evidenced by high melting point of solids
* **Giant covalent lattices**
* covalent bonds between atoms are very strong, difficult to overcome, evidenced by very high melting point of solids
* all electrons are localised in bonds and are not free to move away, evidenced by no electrical conductivity in the solid
* **Covalent molecules**
* if a solid forms then the molecules are at the lattice points with intermolecular forces of attraction between the molecules
* on melting it is not the covalent bonds inside the molecule that are broken but the attractive forces between the molecules which are overcome, evidenced by relatively low melting points of solids
* no free electrons that can move off the molecule, they are localised in bonds, evidenced by solids of covalent molecule material not conducting electricity
37
New cards
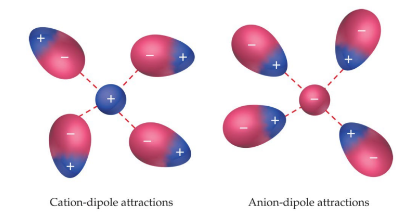
Ion-dipole
* Ion-dipole interactions are an important force in solutions of ions.
* The strength of these forces are what make it possible for ionic substances to dissolve in polar solvents.
* The strength of these forces are what make it possible for ionic substances to dissolve in polar solvents.
38
New cards
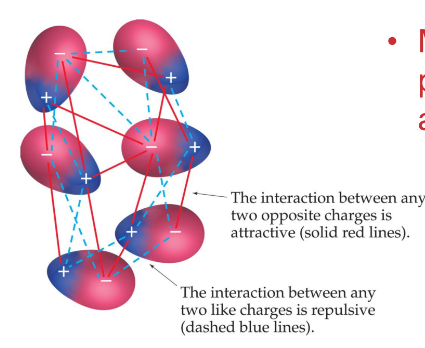
Dipole-Dipole
Molecules that have permanent dipoles are attracted to each other.
* The positive end of one is attracted to the negative end of the other and vice- versa.
* These forces are only important when the molecules are close to each other.
* The positive end of one is attracted to the negative end of the other and vice- versa.
* These forces are only important when the molecules are close to each other.
39
New cards
Hydrogen bonding
* The dipole-dipole interactions experienced when H is bonded to N, O, or F are unusually strong.
* We call these interactions hydrogen bonds.
* We call these interactions hydrogen bonds.
40
New cards
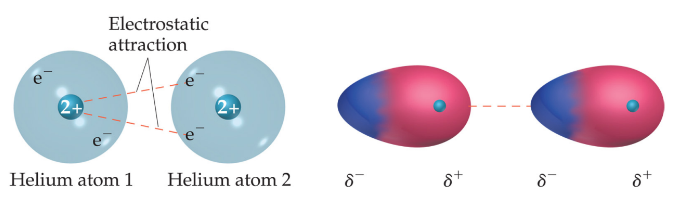
London dispersion forces
* Attractions between instantaneous + induced dipole
* Instantaneous dipole occurs when an electron cloud is slightly skewed towards 1 direction
* Applies to any atom
* Very weak
* Increased with size
* Instantaneous dipole occurs when an electron cloud is slightly skewed towards 1 direction
* Applies to any atom
* Very weak
* Increased with size
41
New cards
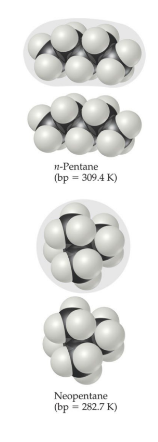
Factors affecting London dispersion forces
* The shape of the molecule affects the strength of dispersion forces: long, skinny molecules (like n-pentane tend to have stronger dispersion forces than short, fat ones (like neopentane).
* This is due to the increased surface area in n-pentane.
* This is due to the increased surface area in n-pentane.
42
New cards
Which Have a Greater Effect: Dipole-Dipole Interactions or Dispersion Forces?
* If two molecules are of comparable size and shape, dipole-dipole interactions will likely be the dominating force.
* If one molecule is much larger than another, dispersion forces will likely determine its physical properties.
* If one molecule is much larger than another, dispersion forces will likely determine its physical properties.
43
New cards
IMF Physical properties - Viscosity
Resistance of a liquid to flow is called **viscosity**.
It is related to the ease with which molecules can move past each other.
* Viscosity increases with stronger intermolecular forces and decreases with higher temperature.
It is related to the ease with which molecules can move past each other.
* Viscosity increases with stronger intermolecular forces and decreases with higher temperature.
44
New cards
IMF Physical properties - Surface tension
Surface tension results from the net inward force experienced by the molecules on the surface of a liquid.

45
New cards
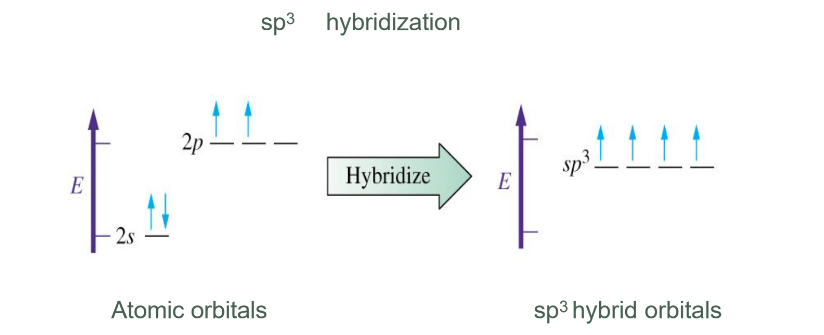
Hybridisation
In chemistry, hybridization is the concept of mixing atomic orbitals to form new hybrid bonding orbitals, which can form covalent bonds between atoms, or hold a lone pair of electrons, in valence bond theory.
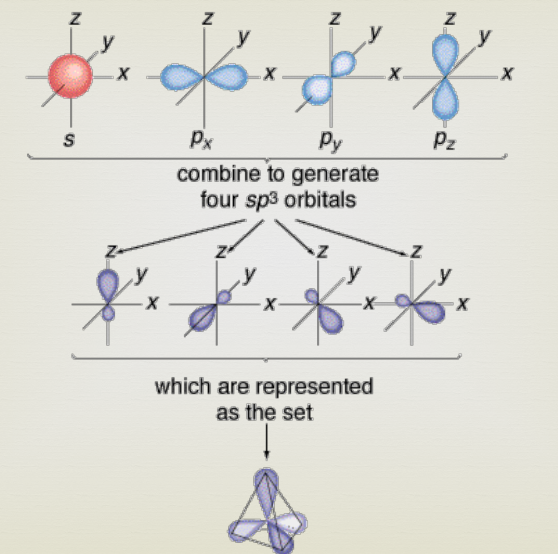
46
New cards
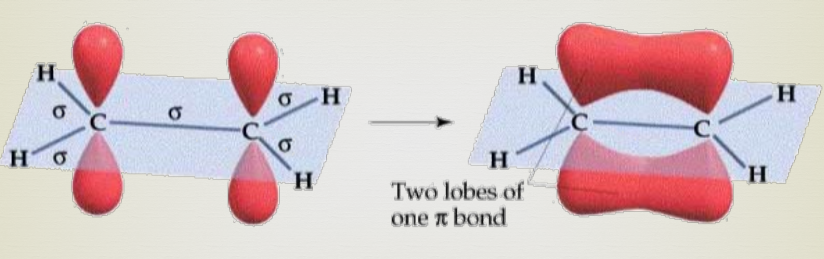
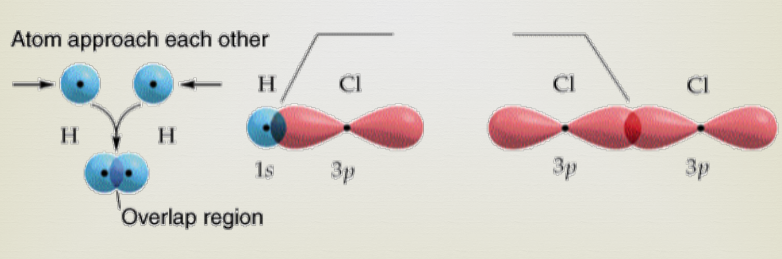
Sigma bonds
Sigma bond (s) → A bond where the line of electron density is concentrated symmetrically along the line connecting the two atoms.
47
New cards
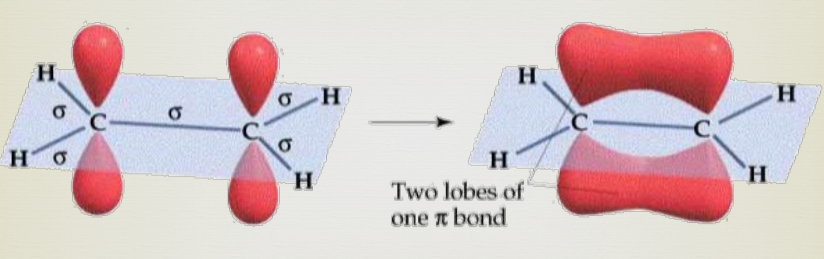
Pi bonds
A bond where the overlapping regions exist above and below the internuclear axis (with a nodal plane along the internuclear axis).