Biological molecules
1/99
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
100 Terms
How are molecules bound to haemoglobin
The presence of the haem group (and Fe2+) enables small molecules like oxygen to be bound more easily
This is because as each oxygen molecule binds it alters the quaternary structure (due to alterations in the tertiary structure) of the protein which causes haemoglobin to have a higher affinity for the subsequent oxygen molecules and they bind more easily
(Fe2+) in the prosthetic haem group also allows oxygen to reversibly bind as none of the amino acids that make up the polypeptide chains in haemoglobin are well suited to binding with oxygen
What is the function of haemoglobin
Responsible for binding oxygen in the lungs and transporting the oxygen to tissue to be used in aerobic metabolic pathways
as oxygen isn't very soluble so it can be carried around the body more efficiently when bound to haemoglobin
How does haemoglobin appear bright red
The prosthetic haem group contains an iron II ion (Fe2+) which is able to reversibly combine with an oxygen molecule forming oxyhaemoglobin and results in the haemoglobin appearing bright red
Why is the arrangement of R-groups in haemoglobin important to the functioning of haemoglobin
how is this relevant to sickle cell disease
If changes occur to the sequence of amino acids in the subunits this can result in the properties of haemoglobin changing
This causes SCA
where base substitution results in the amino acid valine (non-polar) replacing glutamic acid (polar) making haemoglobin less soluble
Outline the structure of haemoglobin
their polar hydrophilic R groups orientate themselves on the outside of the protein which are globin proteins
2x alpha global 2x beta globin (each subunit has a prosthetic harm group)
The four globin subunits are held together by disulphide bonds and arranged so that their hydrophobic R groups are facing inwards and the hydrophilic R groups are facing outwards
this preserves the 3D spherical shape
and also helps maintain its solubility
Each haemoglobin with the four haem groups can therefore carry four oxygen molecules (eight oxygen atoms)
Why do globular proteins form a spherical shape when folding into their tertiary structure
their non-polar hydrophobic R groups are orientated towards the centre of the protein away from the aqueous surroundings
their polar hydrophilic R groups orientate themselves on the outside of the protein
What is a conjugated protein
outline any other features about them and prothetic groups
A protein that contains a non-protein chemical group such as a prosthetic group or cofactor
some globular proteins are conjugated
A prothetic group is a permanent non-protein part of a protein molecule
haemoglobin contains the prosthetic group haem
Outline the properties and functions of globular proteins
Compact and soluble in water
The solubility of globular proteins in water means they play important physiological roles as they can be easily transported around organisms and be involved in metabolic reactions
The folding of the protein due to the interactions between the R groups results in globular proteins having specific shapes
Thus, enabling globular proteins to have physiological roles, e.g enzymes can catalyse specific reactions and immunoglobulins (antibodies) can respond to specific antigens
What are the properties of haemoglobin
NOMAPS
No. of polypeptides
4 - 2x alpha global and 2x beta globin
Outline (shape)
Spherical, round
Main function
Functional (transport of oxygen)
Amino acid variation
Variable
Prosthetic group?
Yes, haem group
Solubility
Soluble in water
What are the properties of collagen
NOMAPS
No. of polypeptide chains
3 - triple helix
Outline (shape)
Long and thin
Main function
structural
Amino acid variation
Repetitive - every third amino acid is glycine
Prosthetic group?
none
Solubility
insoluble in water
What are the functions and properties of collagen
flexible structural protein forming connective tissues
the presence of many hydrogen bonds within the triple helix structure of collagen results in great tensile strength
this enables collagen to be able to withstand large pulling forces without stretching or breaking
the staggered ends of the collagen molecules within the fibrils provide strength
stable protein due to the high proportion of proline and hydroxyproline amino acids
these amino acids increase stability as their R groups repel each other
The length of collagen molecules means they take too long to dissolve in water (insoluble)
What are the properties of globular proteins
SPANDS
Shape
Round and spherical
Purpose
Functional
Acid sequence
Irregular amino acid sequence
Durability
more sensitive to change in pH and temp
Solubility
Generally soluble in water
Outline the properties of fibrous proteins
SPANDS
Shape
Long and narrow strands
Purpose
Structural
Acid sequence
Repetitive amino acid sequence
Durability
less sensitive to change in pH and temp
Solubility
Generally insoluble in water
What are three common fibrous proteins and their functions
Keratin
compose fingernails, horns and hair
Collagen
connective tissue found in skin, hair and tendons
Elastin
found in connective tissue, tendons, skin, bone and artery walls
Why are fibrous proteins good for structural roles
Their insolubility as well as its very organised structure make these proteins suitable for structural roles
Outline the features of fibrous proteins
Long strands of polypeptide chains that have cross-linkages due to H bonds
These proteins have little or no tertiary structure
Insoluble in water due to a large number of hydrophobic R-groups
Fibrous proteins have a limited number of amino acids with the sequence usually being highly repetitive
What are some examples of quaternary proteins
Haemoglobin - made of 4 subunits (2 alpha, 2 beta and held together by disulphide bridges)
Collagen
Insulin - Weak interactions between the subunits in the insulin polypeptide help to stabilise the overall quaternary structure
Outline the Quaternary structure of proteins
Multiple 3D polypeptides can come together to form a complex, quaternary structure (same a tertiary)
also involves the interaction/bonding between R-variable side groups of amino acids in separate polypeptide chains
Describe the hydrogen bonds between amino acids
Hydrogen bonds also form between amino acids
Hydrogen bonds are relatively weak but when there are many, the overall stability of the tertiary structure increases
Describe the disulphide bridges in amino acids
what type of bonds are they and what proteins do they contain
These are covalent bonds set up within proteins containing cysteine (CYS) amino acids
often help proteins resist denaturing
Describe ionic bonds in amino acids
Charged amino acids have a positively or negatively charged ion in their side chain
Charged amino acids can form relatively strong ionic bonds with other charged amino acids
Ionic bonds between amino acids are quite rare
Outline the tertiary structure of proteins
Interactions between R groups creates the complex 3D tertiary structure of a protein
The 3D structure is usually coiled or folded
Bonds are either hydrophilic, ionic or disulphide
Outline the secondary structure of proteins
how is the structure determined
what else can it form
Amino acids in a polypeptide chain can form hydrogen bonds between other amino acids within the chain
The hydrogen bonds cause the protein to fold into specific structures
Folding of the polypeptide determines its secondary structure
Can form beta-plated sheets or an Alpha Helix
Outline the primary structure of proteins
what determines the primary structure of polypeptides
what determines the sequence of amino acids
the sequence of amino acids in a polypeptide chain determines the structure of the polypeptide
amino acids are polymerised by condensation reactions to form peptide bonds
determined by the gene encoding the protein
What are peptide bonds formed between
two amino acids
an amino group and a carboxyl group
How are peptide bonds digested
Hydrolisis
What is a Peptide formed by
Condensation reaction
What are ester bonds formed between
Glycerol and fatty acids
A carboxyl group and a hydroxyl group
How are ester bonds digested
Hydrolisis
How are ester bonds formed
Condensation reactions
What are glycosidic bonds formed between
two monosaccharides
hydrogen and a hydroxyl group
How are glycosidic bonds digested
Hydrolisis
The two functional groups in amino acids are
The carboxyl group (COOH)
The amino group (NH2)
Describe how peptide bonds are formed
When two amino acids react together, a bond forms between the carboxyl group of one amino acid and the amino group of a second amino acid
One water molecule is released as a by-product
The bond formed between two amino acids is a covalent bond called a peptide bond
What is a peptide bond
A peptide bond is a chemical bond that links two amino acids together in a protein. It forms when the carboxyl group of one amino acid reacts with the amino group of another, releasing a molecule of water (H₂O).
Outline the structure of polypeptides
Polypeptides are made from chains of amino acids
There are amino acids at each end of the polypeptide chain
These amino acids form the two end terminals:
The N-terminal (amine terminal)
The C-terminal (carboxyl terminal)
What is an R group
The R group is different in each amino acid.
The R group determines how the amino acid interacts and bonds with other amino acids in the polypeptide.
What are glycosidic bonds formed by
Condensation reactions
Outline the structure of amino acids
how do you distinguish an amino acid
Contain Carbon, Hydrogen, oxygen and nitrogen
They are polar and soluble
An amino acid should contain
NH2 (an amino group)
COOH (a carboxyl group)
H (a hydrogen atom)
R (a side group)
How are dipeptides and polypeptides formed
what are bonds between amino acids called
2 amino acids polymerise in a condensation reaction to form water and a dipeptide
Many amino acids can polymerise to form a polypeptide
bonds between amino acids are called a peptide bond
What is the display formula of a generic amino acid
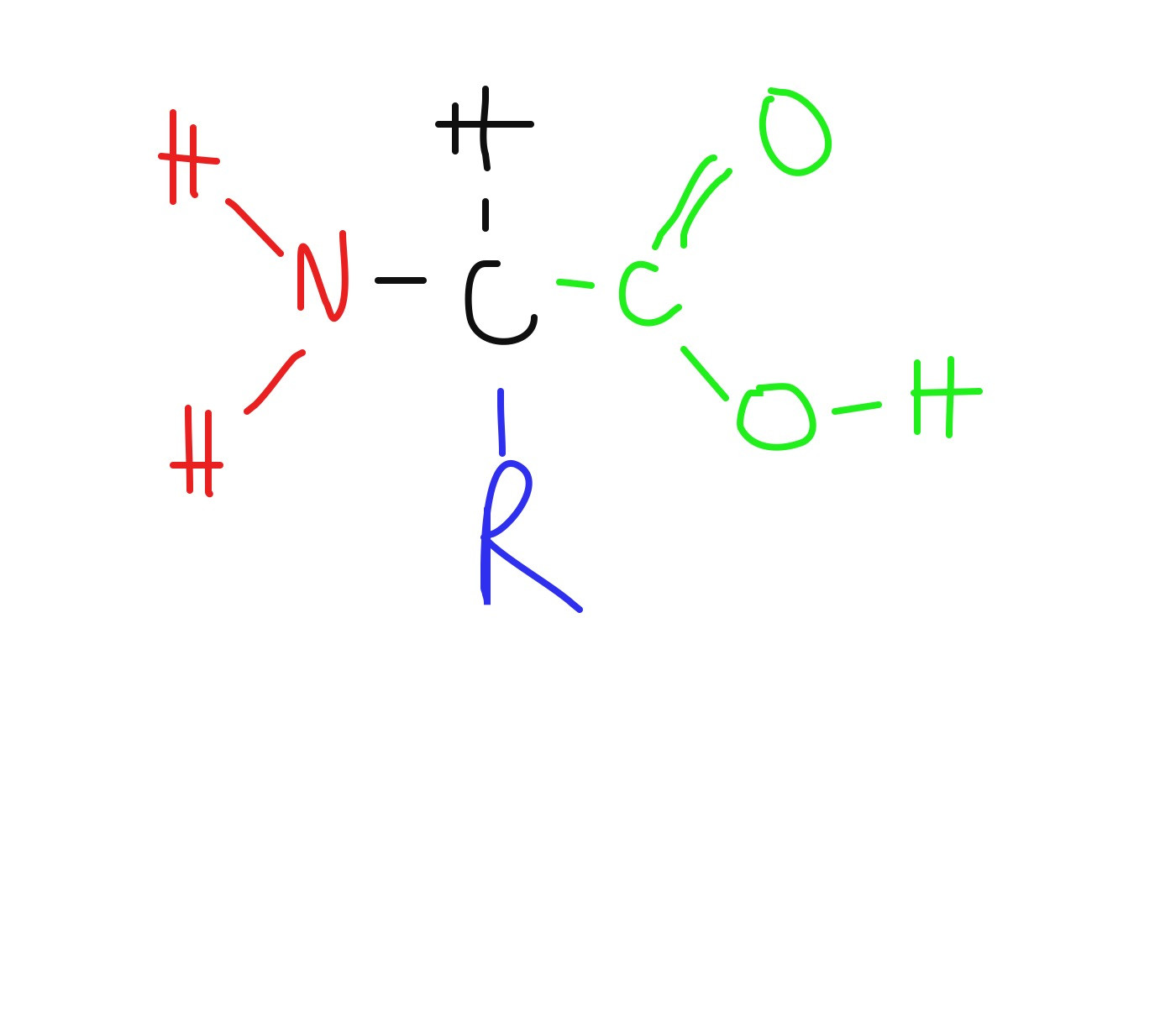
Amino group
Side chain
Carboxyl group
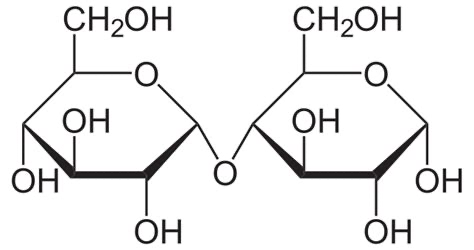
What is the display formula of cellulose
What happens to cholesterol at low temperatures
Cholesterol prevents phospholipid from packing too close together to increase membrane fluidity
What happens to cholesterol at high temperatures
they bind to the hydrophilic tails of phospholipids, causing them to pack together more closely
Makes the membrane more rigid and contain less fluid
Outline the features of Cholesterol
what is its structure
what does it stabilise
Cholesterol is a type of lipid
Cholesterol has a ring steroid structure so it can be converted into steroid hormones (oestrogen and testosterone)
Used to stabilise cell membranes
It's a flat molecule so it can fit between phospholipids
What is Saponification
traditional soaps made using lipids
the lipids (animal or vegetable) are broken down into glycerol and fatty acids
the fatty acids are reacted with the salts of carboxylic acids in a saponification reaction
Outline the features of phospholipids
describe the structure
Special type of lipid that contain phosphate groups and found in biological membranes
Phospholipids aren't considered lipid for triglycerides
have a polar phosphate head (hydrophilic) and a non-polar fatty acid tail (hydrophobic)
Form into phospholipid bilayers to make biological membranes with hydrophilic/polar phosphate heads pointing outwards towards water
Centre of the bilayer is hydrophobic so soluble substances can't pass through
Do unsaturated fatty acids contain a double CC bond
what are they called if they have one CC double bond
what are they called if they have multiple CC double bonds
Yes
1 CC - monounsaturated
multiple CC - polyunsaturated
Do saturated fatty acids contain a double CC bond
No
What is the display formula of triglyceride
what type of bond is in it
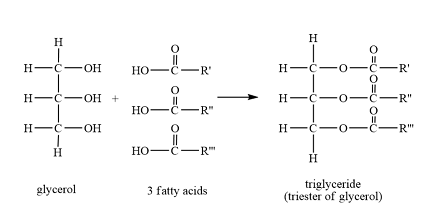
Ester bond
What is the simple structure of a triglyceride
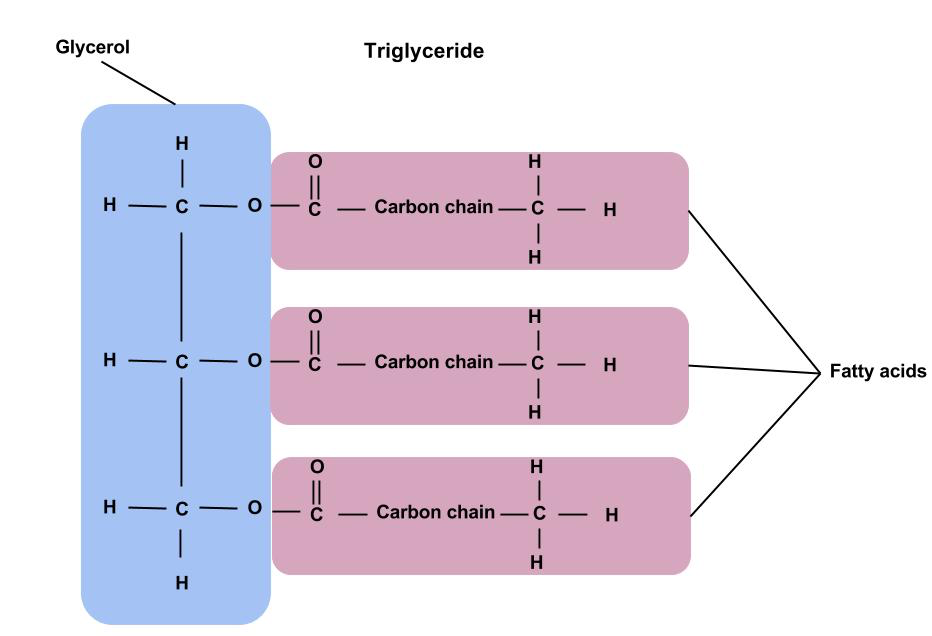
How do biological organisms make use of lipids
strong energy
insulation
waterproofing
protection of foetuses in mammals
Outline the features of Chitin
Chitin is a polysaccharide found in the shells of arthropods (spiders and insects)
Forms the cell walls in fungi and yeast (Murein and Peptidoglycan)
What is a microfibril?
When several cellulose polymers align and hydrogen bonds form between the chains
They are held together by proteins called pectin and carbohydrates called hemicellulose
How do beta glucose molecules orientate in cellulose
The beta-glucose molecules alternate between up and down orientation. This allows several cellulose polymers to align and hydrogen bonds form between the polymer chains to make a structure called a myofibril
Outline the features of cellulose
how many glycosidic bonds does it have
Polymer of the monomer beta glucose
has 1,4 glycosidic bonds only
It forms into straight chains
Large insoluble molecule
Why do we need to store glucose
When glucose dissolves in water (as it's soluble), it causes a low water potential to form (low concentration of water)
Osmosis occurs
Cells/tissue with higher glucose concentration will have water move into them, causing them to become turgid (swell) and then cause tissue damage or kill the cell by lysis (cell bursting)
Outline the features of Glycogen
how many glycosidic bonds does it contain
how rapidly does it hydrolyse
does it have more branches than amylopectin?
Polymer of alpha glucose
Contains 1,4 glycosidic bonds and 1,g glycosidic bonds which make side chains
Large insoluble chains
Rapid to hydrolyse back to glucose as it has many ends that can be acted on by enzymes
Glycogen has more branches and free ends than amylopectin - more compact energy store
stored in liver and muscle cells
What is the display formula of Amylopectin
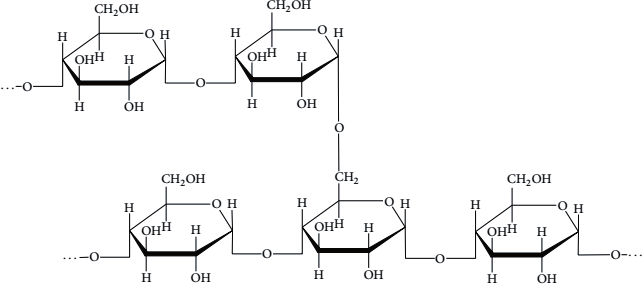
What is the display formula of Amylose
Outline the features of Amylopectin
how many glycosidic bonds does it contain
how fast does it hydrolyse back to glucos
Polymer of Alpha glucose
Contains 1,4 glycosidic bonds and 1,6 glycosidic bonds which make side chains
Large insoluble molecule
compact branched structure with many side chains
Rapid to hydrolyse back to glucose as it has many ends that can be acted on by enzymes
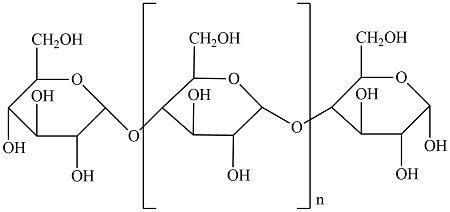
Outline the features of Amylose
how many glycosidic bonds does it contain
what is it a polymer of
how fast does it hydrolyse back to glucose
Polymer of Alpha Glucose
contains 1,4 glycosidic bonds
Large insoluble molecule
forms into a single/unbranched compact coil/helix
slow to hydrolyse back to glucose as it has only 2 ends which can be acted on by enzymes
What are the two types of starch
Amylose and amylopectin
Outline the features of starch
Long chain of glucose
excess glucose during photosynthesis is converted to starch
plants convert starch back into simple sugars at night, as no photosynthesis will occur
How is Water a polar molecule
oxygen atoms in water are slightly negatively charged
hydrogen atoms in water are slightly positively charged
List all of the special properties of water
Metabolic importance
High SHC
High SLH
Cohesive properties
Useful as a solvent
What are the biological properties of water in terms of AQUATIC LIFE
allows organisms to push against it to swim
water creates upthrust on the organisms called buoyancy
water is transparent so animals can see through it to find food or hunt
light can pass through water so it can be absorbed for photosynthesis
water dissolves in oxygen - can be absorbed by marine life via diffusion
water dissolves carbon dioxide - can be absorbed plant and algae for photosynthesis
What are the physical properties of water in terms of surface tension
Water molecules on the surface of water form hydrogen bonds which can support objects on the surface such as water skaters or paperclips
If a detergent is added, this breaks down surface tension
What are the physical properties of water in terms of COHESION AND ADHESION
how is this this important in transport systems
cohesion is where water molecules stick together
Adhesion is when water molecules form hydrogen bonds with other molecules
In the Xylem, water molecules stick to each other and to the walls of the Xylem, allowing the water to be drawn up the Xylem, which also transports nitrate ions and other molecules
This is called Capillary action
How does the density of ice insulate the water bellow, and how does this help living organisms
Ice at 0°C has a lower density than at 4°C meaning it will float on water
This means the surface of the water will freeze but the highest density at 4° will sink to the bottom
This insulates the water bellow
What are the physical properties of water on terms of DENSITY
Density of 998.23 kg/m3 at room temp and pressure
As temp decrease the density increase (like most liquids) until it reaches 1000 kg/m3 at 4°C
When the temp goes bellow 4°C the density starts to decrease
What are the thermal properties of water in terms of SLH
how are these properties useful in a biological context
High SLH of vaporisation (2,269,000 J/kg)
Takes 2,269,000 J/kg of energy to change 1kg of water from liquid to gas with no change in temperature
Utilises evaporation to remove energy such as by sweating
Harder for water to vaporise from living organisms or bodies of water in which they might live or drink from
What are the thermal properties of water in terms of SHC
why are these properties good for biological organisms
has an SHC of 4200 J/kg °C
It takes 4200 J of energy to raise 1kg of water by 1°C
This is because it requires a lot of energy to break the hydrogen bonds between water molecules
this means that water requires a lot of energy to increase in temperature
This is good for biological organisms made mainly of water as they will not change temperature quickly if the environmental temperature increases
What are the chemical properties of water in terms of it as a SOLVENT
Because water is a dipolar molecule, it can form bonds with ions and other polar molecules such as glucose
makes water a universal solvent
both oxygen and carbon dioxide gas can dissolve in water
How would you describe the charge of Hydrogen
what does this look like
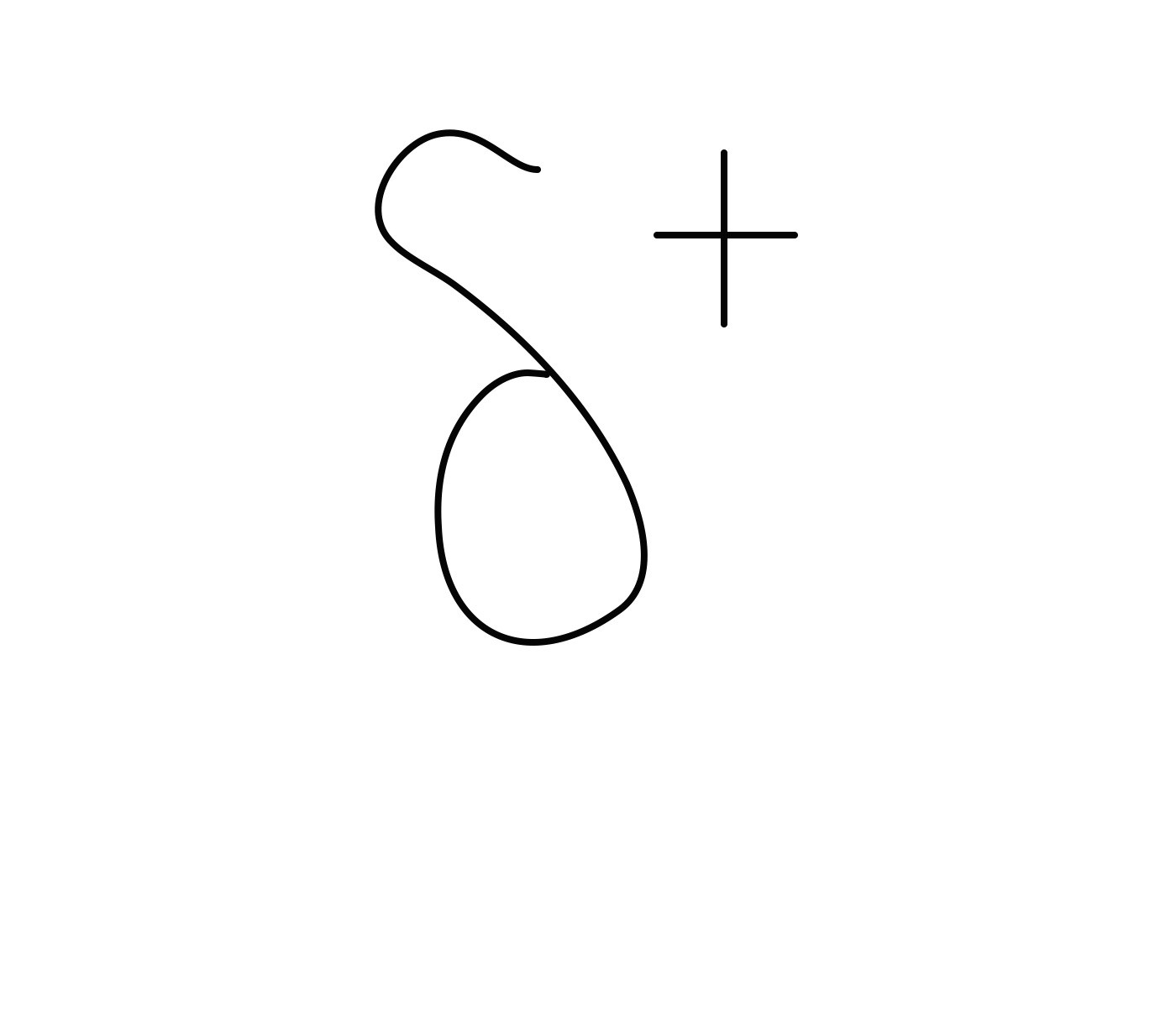
Hydrogen has a slightly positive charge
How would you describe the charge of oxygen
what does this look like
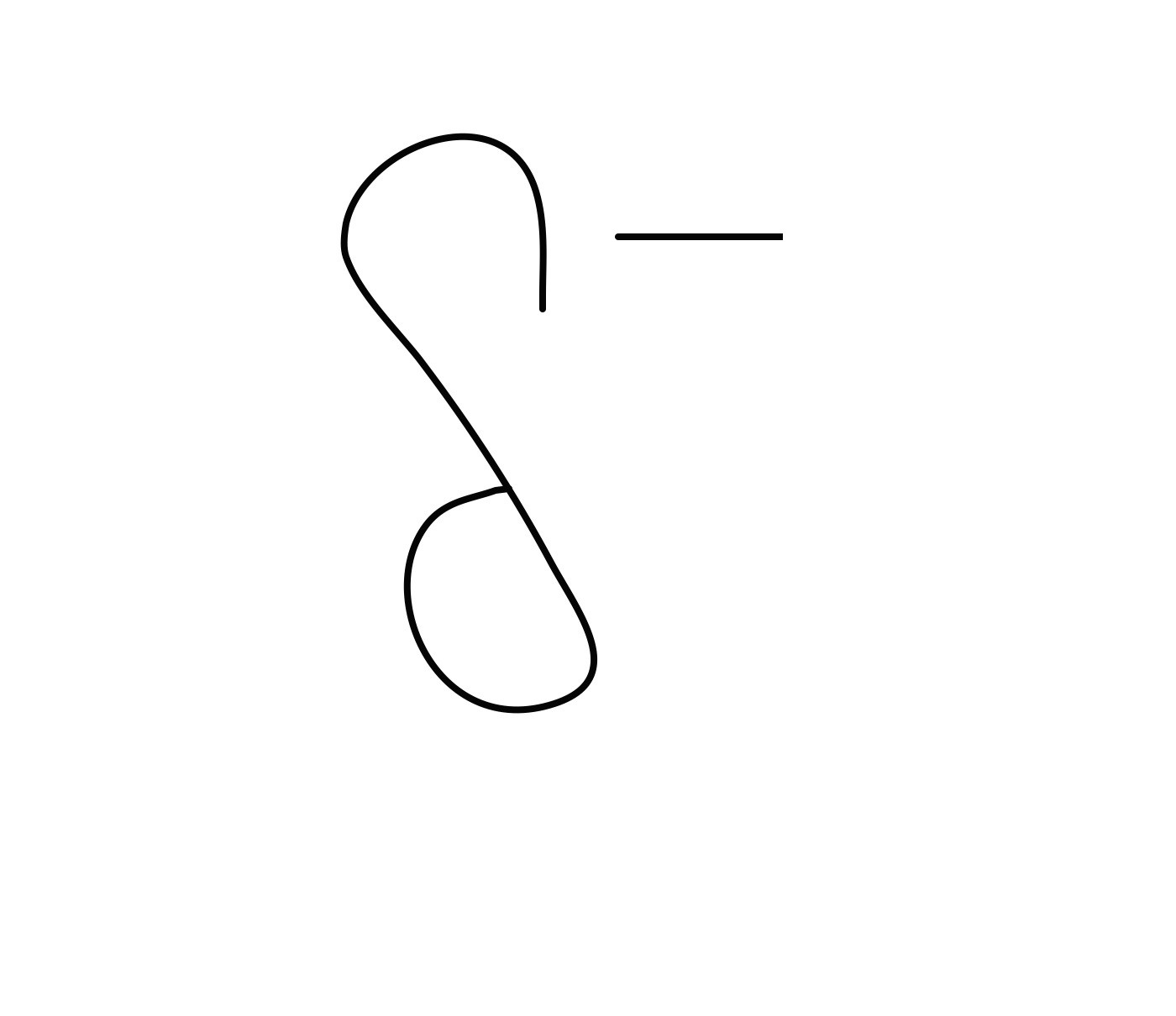
Oxygen has a slightly negative charge
What are the chemical properties of water in terms of DIPOLE
what types of molecules are they
what charges do the ions have
Water molecules are polar
An oxygen atom has more protons in its nucleus than hydrogen, it draws the shared electrons in the covalent bond more towards oxygen
The oxygen has a slight negative charge
The hydrogen has a slight positive charge
The charge isn't fully ionic, but called a dipolar molecule
What are the chemical properties of water in terms of SHAPE
electron configuration
covalent bonding
Oxygen has 6 electrons on its outer shell
Hydrogen atoms have 1 electron on their outer shell
Two O-H covalent bonds form between the hydrogen and oxygen atoms as they share electrons
Two pairs of electrons that aren't involved in covalent bonds repel the other electrons
What does Water look like
display formula
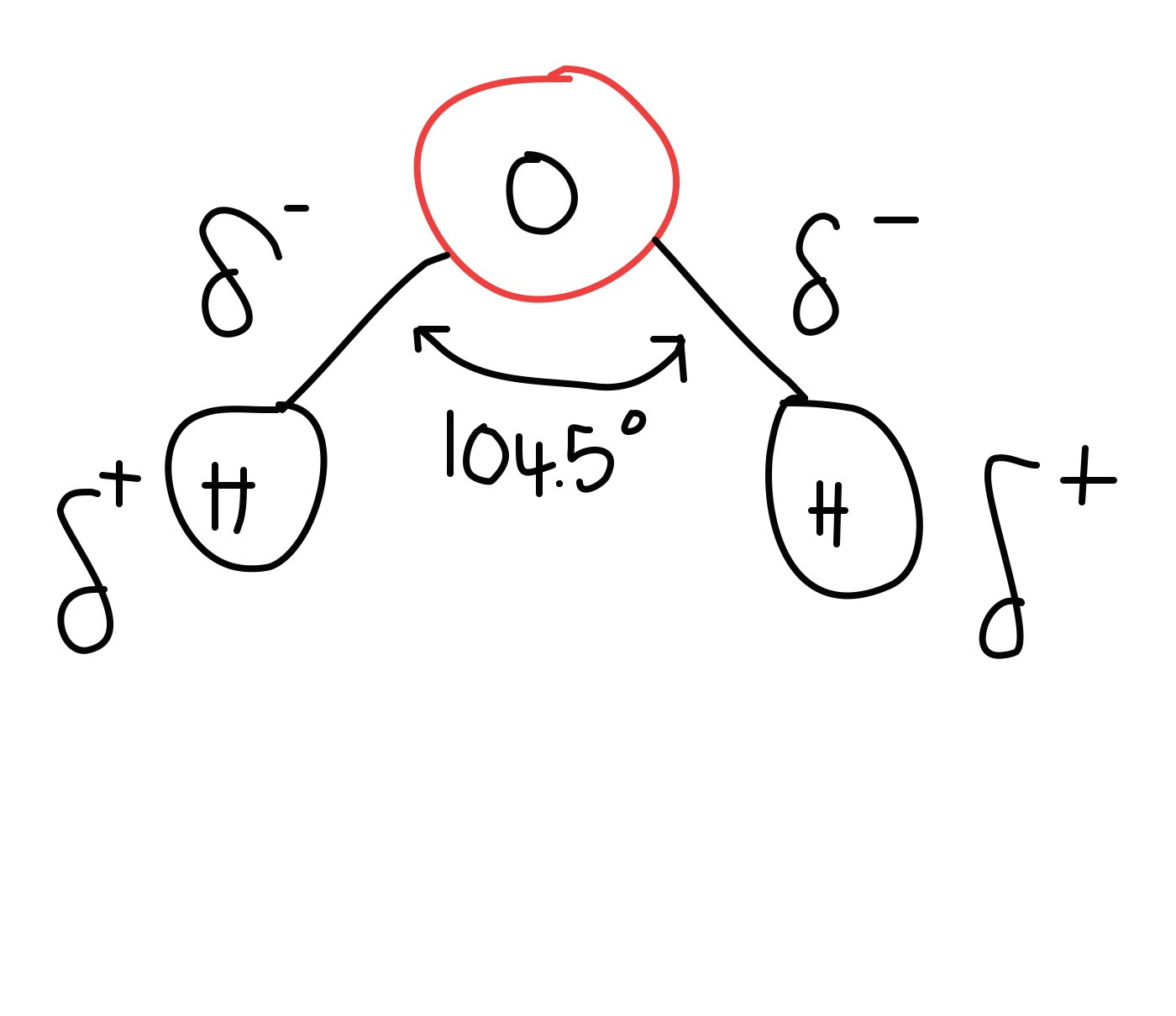
d
What is a pentose carbohydrate
deoxyribose and ribose are both pentose carbohydrates
they have 5 carbons
used in DNA and RNA respectively
What are the features of Lactose
disaccharide
made of glucose - galactose
sugar found in water
produced by lactating mammals (some reptiles as well)
What are the features of sucrose
disaccharide
made of glucose - fructose
table sugar
plants convert glucose to sucrose for transport in the phloem
What are the features of Maltose
Disaccharide
made of Glucose - glucose
product of hydrolysis
involved in the process of malting in brewing and baking
What is a use of galactose
Polymerise to form lactose
monosaccharide
What is a use of fructose
Produced in fruits and nectar
Sweetest sugar
monosaccharide
What is a use of beta-glucose
Polymerised to form cellulose
monosaccharide
What is a use of alpha-glucose
Substrate in respiration
monosaccharides
Polymerised to form Maltose and Starch
Combined with nitrates to form amino acids
Outline the hydrolysis of Maltose
can be split into two glucose molecules
Maltose + water ---> Glucose + Glucose
It can be done either way using 1M Hal at 60°C or an enzyme (maltose)
What are isomers
examples
Isomers have the same molecular formula but a different arrangement of atoms in space
Alpha glucose
Beta glucose
Is glucose a hexose or pentose sugar
what does this mean
Glucose is a hexose sugar
Each molecule of glucose has 6 carbon atoms
What are Oligosaccharides
Oligosaccharides are made of 3-25 monosaccharides and are used as cell surface antigens
What are polysaccharides
Polysaccharides are made from 100+ monosaccharides polymerised into a lay chain
What are Disaccharides
how are they made
Disaccharides are made two monosaccharides via a condensation reaction
What are monosaccharides
Monosaccharides are a monomer from which larger carbohydrates can be made
What are the elements found in nucleic acid
Carbon
Hydrogen
Oxygen
Nitrogen
Phosphorus
What are the elements found in Proteins
carbon
hydrogen
oxygen
nitrogen
sulfur
What are the elements found in carbohydrates
oxygen
carbon
hydrogen
How do you differentiate between Alpha and Beta glucose
(molecule diagram)
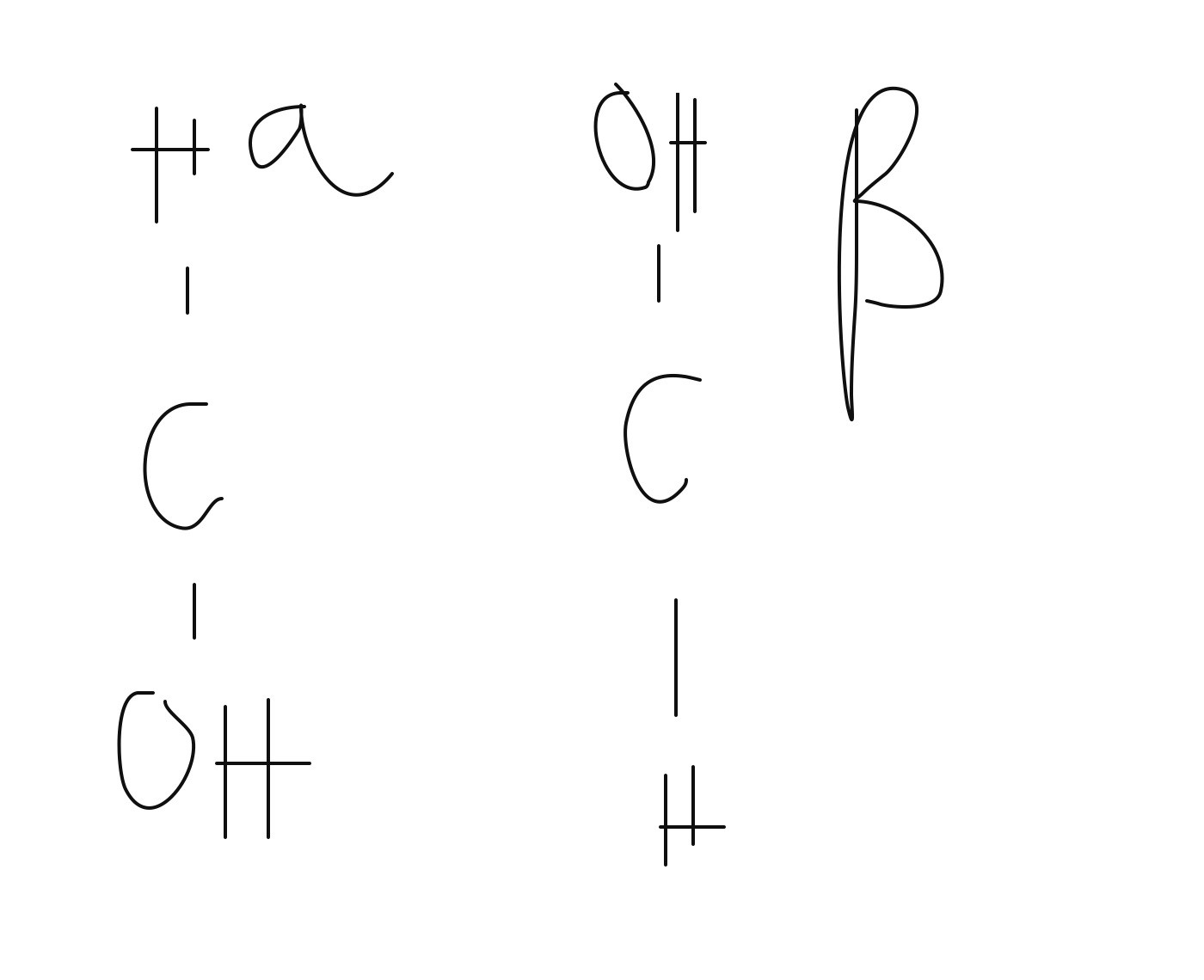
The configuration of an alpha molecule will have OH below the Carbon atom
The configuration of a beta molecule will have OH above the Carbon atom
What molecule is this?
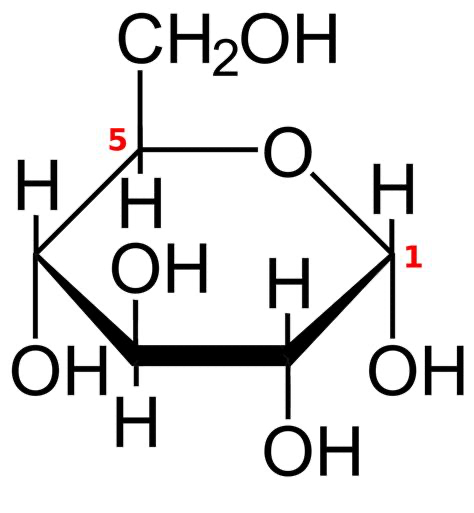
Alpha glucose
What molecule is this?
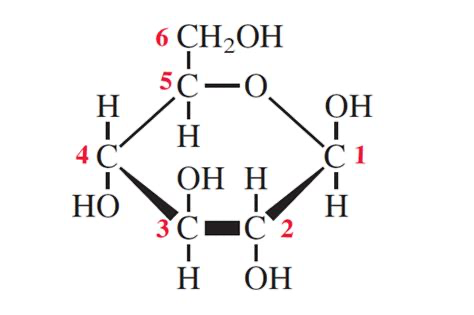
Beta glucose