Ch12 Membrane Structure and Function
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
What makes a good membrane?
selective barrier - lets some things through, blocks others; identifies self from non-self
flexible structure - can bend without breaking
self-assembling - forms spontaneously in water
asymmetric - inside down not = outside
dynamic - components can move around
fluid mosaic model
many different types of molecules all come together to make the whole
Amphipathic lipids
this is why they form spontaneously
hydrophilic head
hydrophobic tail
Why do membranes form spontaneously?
water molecules are highly ordered around hydrophobic substances
entropy increases when hydrophobic tails cluster together
no covalent bonds needed - it’s all about thermodynamics
result: lipid bilayer formation is energetically favorable
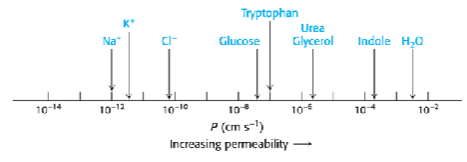
Membrane permeability rules: what can cross easily?
small, nonpolar molecules (O2, CO2)
small, uncharged polar molecules (H2O)
Membrane permeability rules: what is blocked?
ions (Na+, K+, Cl-) high energy cost to remove water shell
large polar molecules (glucose, amino acids)
charged molecules
hydrophobicity and size determine permeability of molecules
Rank these molecules from most to least likely to cross a lipid bilayer easily:
• Sodium ion (Na+)
• Ethanol (CH3CH2OH)
• Glucose (C6H12O6)
• Carbon dioxide (CO2)
CO2, CH3CH2OH, C6H12O6, Na+
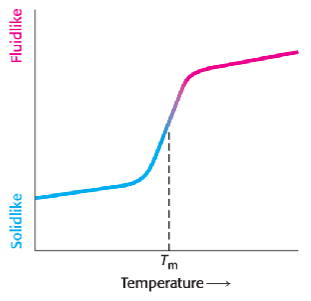
Membrane fluidity: the goldilocks principle
too fluid = problems
membrane loses integrity
proteins can’t function properly
too rigid = problems
membrane can crack
transport processes shut down
just right = functional
flexible enough for protein function
stable enough for barrier function
decreasing melting temperature means it will be fluid for longer (shift left on curve) making it more permeable; vice versa
Fatty acid composition: saturated fats
more rigid
straight chains packed tightly
higher melting temperature
Fatty acid composition: unsaturated fats
more fluid
kinks from double bonds prevent tight packing
lower melting temperature
cis unsat will increase fluidity
trans double bonds will look and act like the fully saturated fatty acids because they do not introduce kinks
Controlling membrane fluidity
chain length: longer = more rigid, shorter = more fluid
cholesterol: acts as a fluidity buffer in animal cells
Choose: Cholesterol will increase/decrease fluidity at lowered temperatures and will increase/decrease fluidity at higher temperatures.
increase; decrease
Scenario: bacteria living in hot springs (80 C) vs arctic bacteria (0 C)
Question: how would their membrane lipid composition differ to maintain proper fluidity
think about what chains would help each environment
long saturated chain to counter the hot spring
higher degree of CIS unsaturation of short chains for cold
hot: things are moving around too much so we want to increase the number of hydrophobic interactions via increasing chain length (increase number of atoms)
Cholesterol: fluidity buffer
high temperature: restrains phospholipid movement, decreasing fluidity
low temperatures: prevents membranes from becoming too rigid (crystalline), increasing fluidity
Effect of cholesterol on different membrane composition
saturated membranes: tends to disrupt packing and introduce spacing, increasing fluidity
unsaturated membranes: cholesterol tends to fill gaps created by kinds in unsaturated chains, decreasing fluidity
Cholesterol-like molecules in other species
bacteria: hopanoids
plants: sterols
There are four lipid bilayers composed of varying ratios of saturated fatty acids, unsaturated fatty acids, and cholesterol. Assume all bilayers are at the same, physiological temperature
Membrane A: high saturated, low unsaturated, no cholesterol
Membrane B: high unsat, low sat, no chol
Membrane C: high sat, low unsat, high chol
Membrane D: high unsat, low sat, high chol
Rank the membranes from most fluid to least fluid under these conditions
B > D > C > A
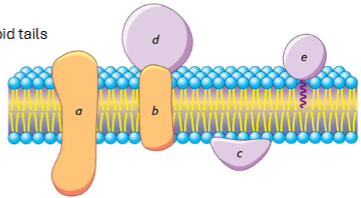
2 main categories of membrane proteins: integral proteins
embedded in or spanning the membrane
have hydrophobic regions that interact with lipid tails
ex. ion channels, transporters
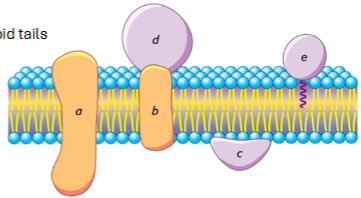
2 main categories of membrane proteins: peripheral proteins
associated with membrane surface
easily removed from membrane
usually interact with polar head groups
protein structure determines membrane association
Passive transport
no energy needed - move down gradient (high conc. to low conc.)
simple diffusion, facilitated diffusion (aquaporins for H2O)
Active transport
energy required; move against gradient
primary active transport (ATP), secondary activate transport (using energy gradient set up by something else)
proteins make membranes selectively permeable
Scenario: a cell needs glucose when internal glucose is already higher than external glucose
is the glucose trying to move with or against its gradient?
what type of transport is needed
what energy source would be required?
how might the cell accomplish this?
against
active
ATP
protein transporters
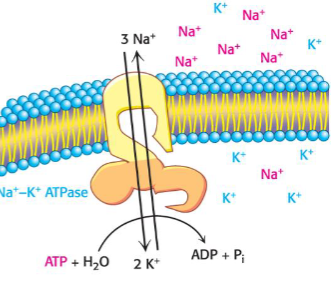
Primary active transport: Na+/K+ pump
pumps 3 Na+ out and 2K+ in per ATP
maintains membrane potential
drive secondary transport processes
uses ~30% of cell’s total energy
essential for nerve function
target of important drugs (digitalis)
mechanism: P-type ATPase with phosphorylation intermediate
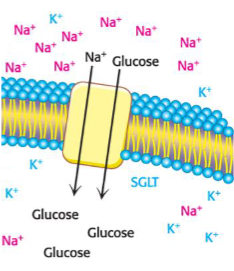
Secondary active transport: Na+/Glucose symporter
uses one gradient to drive transport of another molecule
Na+ gradient powers glucose uptake
primary pumps create gradients that power secondary transport
antiporter - Na+ drives Ca2+ removal
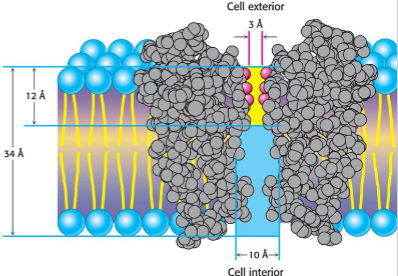
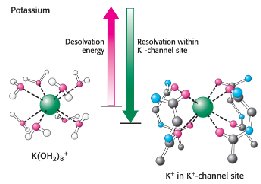
Ion channels
highly selective: specific ion types only
extremely fast: millions of ions per second
regulated: can open and close (gated)
ex. K+ channel
selectivity filter perfectly fits K+ ions
change repulsion creates rapid transport
demonstrates structure function relationship
Clinical insight: what is Tetrodotoxin (TTX)?
potent neurotoxin produced by pufferfish (fugu)
also found in blue ringed octopus, some frogs, and bacteria
lethal dose: 1-2 mg for humans
how does TTX works?
it selectively blocks voltage-gated Na+ channels
binds to channel opening and physically plugs the pore
prevents Na+ influx —> blocks nerve depolarization and signal propagation
results in paralysis and respiratory failure
You discover bacteria in a high-salt environment. Predict three membrane-related adaptations they might have and explain why each would be beneficial.
cell needs to counteract salt in and H2O out
osmotic pressure - high salt will try to get rid of H2O (crenation)
too much salt is coming in
downregulate aquaporin to prevent H2O out
cell might adapt to exploit the salt gradient —> 2nd transport, brings in something like glucose or push out what it doesn’t need
upregulate active transport, push more salt out like Na+/K+