2c - Periodic Properties
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
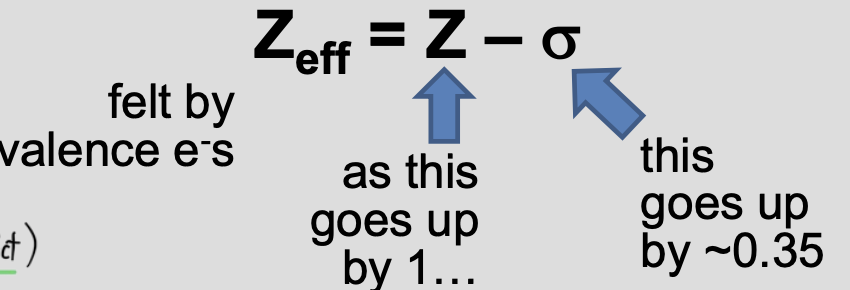
Effective Nuclear Charge
Zeff is the net positive charge experienced by a valence e-, accounting for both nuclear attraction and shielding.
⬆Zeff= less shielding = more nuclear attraction
⬇Zeff= more shielding = less nuclear attraction
Periodic trend of Zeff
✦ Increases across a period because nuclear charge Z increases while same-shell e- only partially shield
✧ Slightly increases down a group, but additional core e- shells significantly increase shielding (less nuclear attraction)
Periodic trend of Ionization Energy
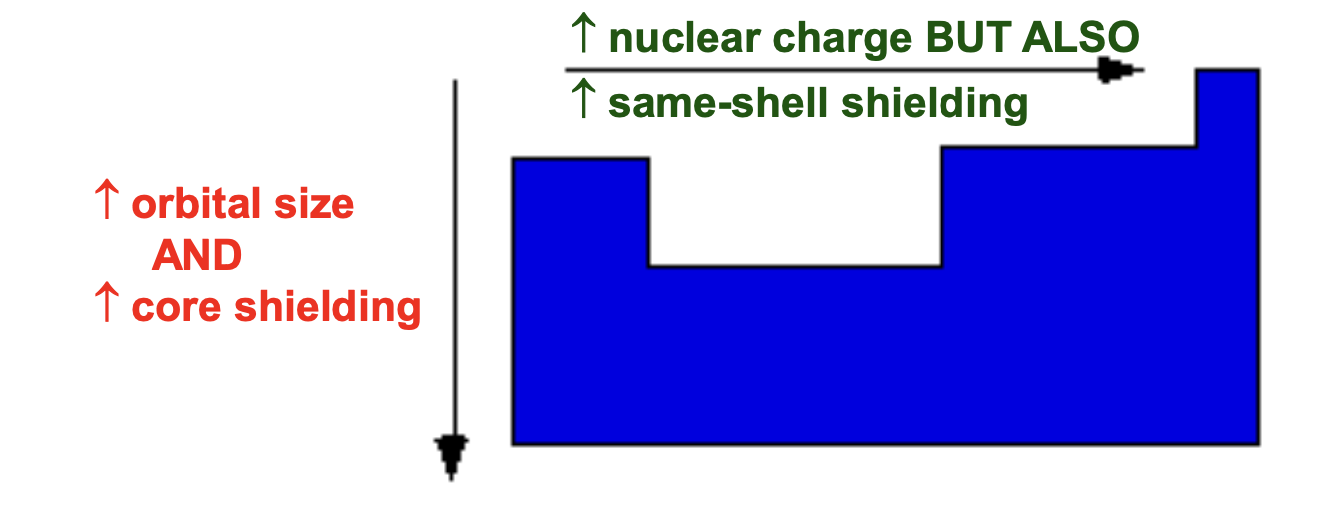
Ionization energy (IE) = the energy required to remove an e- from an atom
✧ Decreases down a group because the e- being removed successively larger shells.
✦ Increases across a period because of incomplete screening from other e-
Ionization energy trend exceptions
✦ discontinuity between ns2np0 & ns2np1: 2s penetrates closer than 2p = 2s in lower in E = need more E to eject e- form 2s
✦ discontinuity between np3 & np4 (adding a 4th e- to a ½-filled shell): maximizing exchange E 𝚷e while relieving pairing E 𝚷c
∆E
The stabilizing energy gained when electrons with parallel spins occupy degenerate orbitals. The lower the value, the more stable
𝚷c
Energy required to pair two electrons in the same orbital, overcoming their repulsion. Less pairing E increases stability
𝚷e
Exchange energy. Stabilizing energy gained when electrons with parallel spins occupy degenerate orbitals. This lowers the total energy ∆E and increases stability.
Periodic trend of Electron Affinity
EA = the energy of adding of an electron to a neg. ion
✧ Increases (more -) across a period due to increasing Zeff and stronger nuclear attraction
✧ Decreases (less -) down a group due to increased atomic size and shielding = e- addition less favoured
**less E is needed to remove e- from an anion because of higher shielding
Periodic trend of Electronegativity
EN - the tendency of an atom to attract bonding e-
✦ Increases across a period due to higher Zeff (e- pulled together = smaller atomic radius)
✧ Decreases down a group due to increased atomic size (e- are farther from nucleus) and shielding. This decrease is more marked in groups on the right side
**𝛘 of noble gases is “irrelevant” as they aren’t very reactive
Electronegativity scales
✧ Mulliken Scale - atom has high 𝛘 if it has high IE and/or high +EA
✧ Allen Scale - designed for the main group elements, uses ionization enthalpy data (∆HIE)
✧ Allred Rochow Scale - considers the Zeff experienced by a bonding e-.
✧ Pauling Scale - considers the difference in bond strength between a bond’s real strength and its calculated “non-polar” strength
Periodic trend of Atomic Radii
✦ Atomic radius increases down a group as new e- shells are added
✧ Decreases across a period due to increasing Zeff (⬆Z and only partial shielding by same ns,np e-)
Coordination number
# of attachments between a metal and its ligands
Ionic Radii
Ions get larger going down a group, with increasing coordination number
✦ cations are smaller than parent ions: fewer e-, higher Zeff, e- are held tighter
✦ anions are larger than parent atoms: more e-, lower Zeff, e- held looser
✧ isoelectronic species decrease in size as Z increases: same shielding but higher Z = higher Zeff = e- are pulled closer (O2->F->Na+>Mg2+)
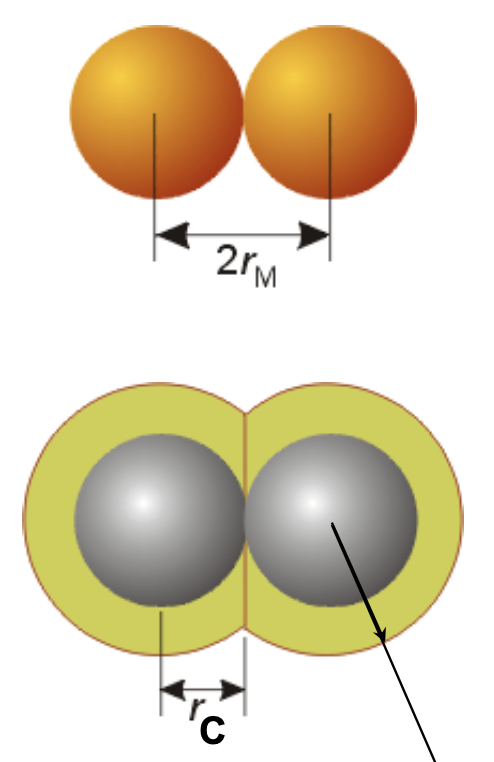
Metallic radius v.s. Covalent radius
Metallic radius: ½ the distance between the nuclei of metal atoms in the solid state
Covalent radius: ½ the distance between identical atoms in a covalent compound
These 2 radii = atomic radii
Lanthanide Contraction
The gradual decrease in atomic radii across the lanth. series due to poor shielding by 4f e-
✦ similar sizes between 6th and 5th row transition metals
✦ 6th row d-block transition metals are smaller than “expected”