Intermolecular Forces Review (AP Chemistry)
Intermolecular forces (IMFs)
Intermolecular forces (IMFs) are the forces that attract or repel entire molecules. These forces arise from differences in charge between molecules. Many students mistakenly confuse IMFs with intramolecular forces, which are the forces that hold atoms together within a molecule.
Intermolecular forces
IMFs are the forces that hold molecules together
Types:
London Dispersion Forces
Dipole-dipole Forces
Hydrogen Bonding
Ion-dipole Forces
Weaker than intramolecular forces
Exist over a larger distance
Coulomb's law: the closer the two particles are, the stronger the attraction

Intramolecular forces
Forces within a molecule that hold atoms together
Types:
Covalent bonds
Metallic bonds
Ionic bonds
Differences between IMFs and intramolecular forces:
Strength: IMFs are much weaker than intramolecular forces.
Distance: IMFs exist over a larger distance than intramolecular forces.
Type of interaction: IMFs involve attractions between entire molecules, while intramolecular forces involve attractions between atoms within a molecule.
Additional notes
The type of IMF present in a molecule depends on its shape, size, and polarity.
IMFs are important for determining the properties of substances, such as boiling point, melting point, and solubility.
Types of Intermolecular Forces:
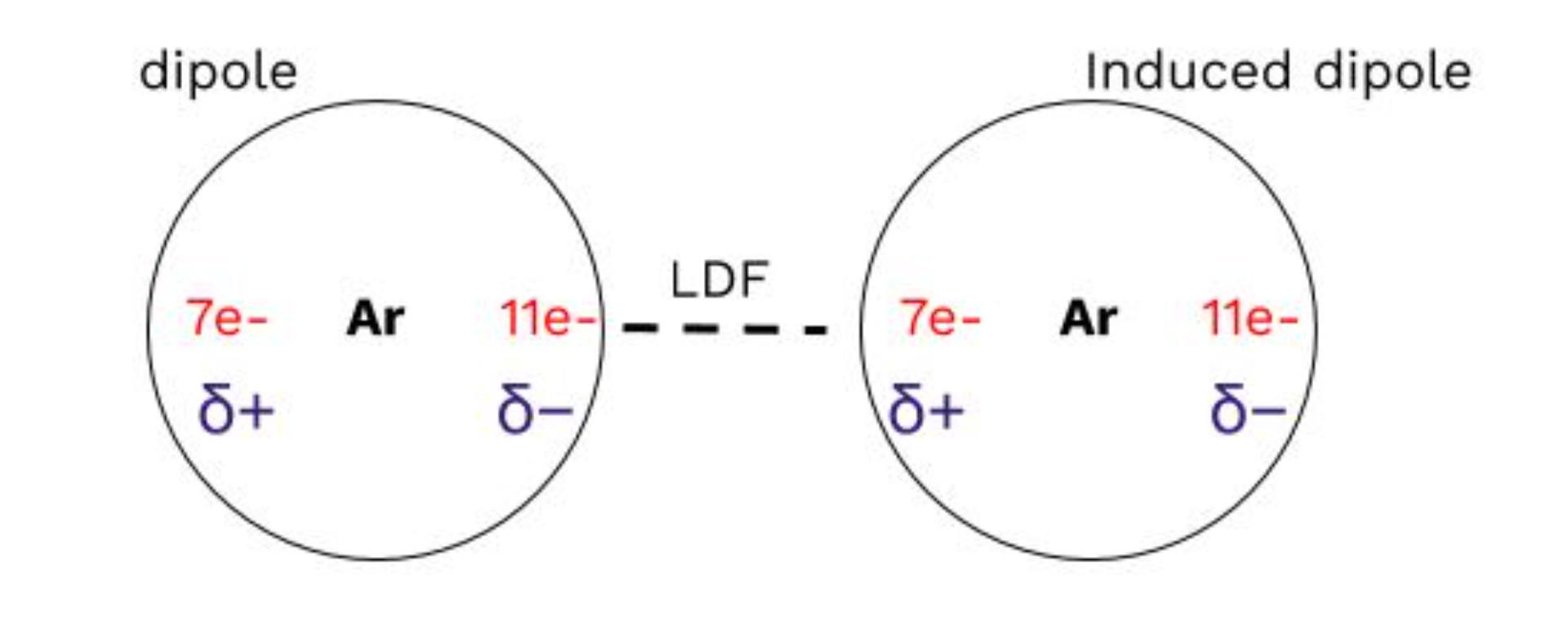
1: London Dispersion Forces (LDFs)
Weakest type of intermolecular force
Present in all molecular substances
Only force between nonpolar molecules and noble gases
Always present alongside other IMFs
How LDFs Work:
Temporary dipoles: Electrons in a nonpolar molecule can temporarily shift to one side, creating a partial positive and partial negative charge.
Induced dipoles: This temporary dipole can induce a similar dipole in a neighboring molecule.
Coulombic interactions: The partial charges on the molecules attract each other, forming a weak LDF.
Factors Affecting LDF Strength:
Molecular size: Larger molecules have more electrons, making them more polarizable and increasing the strength of LDFs.
Polarizability: The ease with which an electron cloud can be distorted. A more polarizable molecule can form stronger temporary dipoles.
AP Exam Tip:
Always include LDFs when identifying intermolecular forces, even if other forces are present.
2: Dipole-Dipole Interactions
Occur between polar molecules
Result from permanent dipoles
Slightly stronger than LDFs
Strength increases with increasing polarity
How Dipole-Dipole Forces Work:
Permanent dipoles: Polar molecules have a permanent separation of charge, with one end slightly positive (δ+) and the other slightly negative (δ-).
Electrostatic attraction: The positive end of one molecule is attracted to the negative end of another molecule.
Coulombic interactions: The strength of the attraction increases as the distance between the dipoles decreases.
Factors Affecting Dipole-Dipole Force Strength:
Polarity: More polar molecules have stronger dipole-dipole forces.
Molecular shape: Molecular shape can influence the orientation of dipoles and the strength of the interaction.
Impact on Physical Properties:
Higher melting and boiling points: Stronger dipole-dipole forces lead to higher melting and boiling points.
Example AP Question:
Answer: Both CS2 and COS experience London Dispersion Forces (LDFs). However, COS, being a polar molecule, also exhibits dipole-dipole forces. Despite LDFs being generally weaker, the larger size of CS2 results in stronger LDFs compared to the combined forces of LDFs and dipole-dipole forces in COS. This stronger intermolecular attraction in CS2 leads to its higher boiling point.
3: Hydrogen Bonding
An unusually strong dipole-dipole attraction between molecules containing hydrogen bonded directly to fluorine, oxygen, or nitrogen (FON).
High electronegativity difference between H and FON atoms leads to strong dipole formation.
Partially positive hydrogen is attracted to the lone pair electrons on FON atoms of neighboring molecules.
Impact on Properties:
High boiling points: Significant energy is required to break these strong intermolecular forces.
Unique properties of water: Responsible for water's high specific heat, cohesion, adhesion, and ability to dissolve many substances.
Stability of biological molecules: Essential for the structure and function of molecules like DNA and proteins.
AP Exam Tip:
On the AP Exam, you might be asked to draw a molecule and show how it forms hydrogen bonds with other molecules. It's important to remember that hydrogen bonds are intermolecular, meaning they occur between different molecules.
4: Ion-Dipole Forces
Attractive forces between ions and polar molecules.
Ions (cations or anions) are attracted to the oppositely charged pole of a polar molecule.
Impact on Properties:
Solubility of ionic compounds: Ion-dipole forces allow ionic compounds to dissolve in polar solvents like water.
Stability of solutions: These forces help maintain the stability of solutions containing ions and polar molecules.
Key Differences
Ion-Ion Attractions
Electrostatic attractions between oppositely charged ions.
Impact on Properties:
High melting and boiling points: Strong ionic bonds require significant energy to break.
Solid crystal lattice structure: Ions are arranged in a regular, repeating pattern.
Formation:
Transfer of electrons between atoms creates ions.
Oppositely charged ions are attracted to each other.
Key Difference from Ion-Dipole Forces:
Ion-ion forces occur within an ionic compound.
Ion-dipole forces occur between an ion and a polar molecule (like water).
Summary of IMFs
Intermolecular Forces (IMFs)
the attractive or repulsive forces that exist between molecules.
Types of Intermolecular Forces
London Dispersion Forces (LDFs)
Weakest type of IMF
Present in all molecules
Caused by temporary dipoles that arise due to the random movement of electrons
Strength increases with increasing molecular size and surface area
Dipole-Dipole Forces
Occur between polar molecules
The positive end of one molecule is attracted to the negative end of another molecule
Strength increases with increasing polarity of the molecules
Hydrogen Bonding
Special type of dipole-dipole force
Occurs between molecules with hydrogen bonded to highly electronegative atoms (F, O, N)
Strongest type of IMF
Responsible for many properties of water
Ion-Dipole Forces
Occur between an ion and a polar molecule
The ion is attracted to the oppositely charged end of the polar molecule
Strongest type of IMF involving a neutral molecule
London Dispersion Forces (LDFs)
Temporary Dipoles:
Electrons in a molecule are constantly moving.
At any given moment, there may be a temporary imbalance of electrons, creating a temporary dipole.
This temporary dipole can induce a dipole in a neighboring molecule.
Weak Attraction:
The temporary dipoles result in weak electrostatic attractions between molecules.
Factors Affecting Strength:
Molecular Size: Larger molecules have more electrons, leading to stronger LDFs.
Molecular Shape: Molecules with larger surface areas have stronger LDFs.
Dipole-Dipole Forces
Permanent Dipoles:
Polar molecules have a permanent separation of charge due to differences in electronegativity.
The positive end of one molecule is attracted to the negative end of another molecule.
Strength of Attraction:
The strength of dipole-dipole forces increases with increasing polarity.
More polar molecules have stronger dipole-dipole forces.
Hydrogen Bonding
Strong Dipole-Dipole Interaction:
Occurs when hydrogen is bonded to a highly electronegative atom (F, O, N).
The highly electronegative atom pulls the shared electrons closer to itself, creating a partial negative charge.
The hydrogen atom, with a partial positive charge, is attracted to the lone pair of electrons on the electronegative atom of another molecule.
Strongest IMF Involving Neutral Molecules:
Hydrogen bonding is significantly stronger than typical dipole-dipole forces.
It plays a crucial role in many biological processes and physical properties of water.
Ion-Dipole Forces
Strongest IMF Involving a Neutral Molecule
Occurs between an ion and a polar molecule.
The ion is attracted to the oppositely charged end of the polar molecule.
The strength of the ion-dipole force increases with the charge of the ion and the polarity of the molecule.
Example:
When NaCl dissolves in water, Na+ ions are attracted to the negative end of water molecules (oxygen), and Cl- ions are attracted to the positive end of water molecules (hydrogen).
Comparing IMF Strengths
Ion-Dipole > Hydrogen Bonding > Dipole-Dipole > London Dispersion Forces
Impact of IMFs on Physical Properties
Boiling Point and Melting Point: Stronger IMFs lead to higher boiling and melting points.
Solubility: Substances with similar IMFs tend to be more soluble in each other.
Surface Tension: Stronger IMFs lead to higher surface tension.
Viscosity: Stronger IMFs lead to higher viscosity.