Chemistry Organic Chemsitry and studying reactions
1/13
Earn XP
Description and Tags
3rd flashcards whoop whoop
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
alkane alkene alkyne
single double triple bonds, hydrocarbon
Alcohol
one hydrogen atom is replaced by -OH
ethane → ethanol
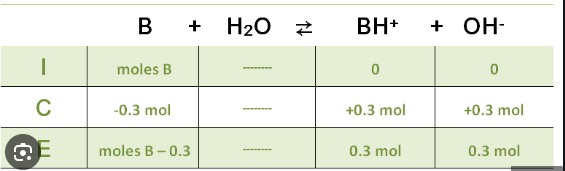
ICE Chart
Polarity
like dissolves like
electrolytes
acids, bases, ionic compounds
Net Ionic Equation
use precipitation to figure out ions and charges, leaving spectator ions alone (don’t react)
Acids
donate hydrogen and produces an anion/ H+
Bases
recive hydrogen and produces OH-/cation
Net Ionic Equation for nuetralization
OH- + H+ = H2)
Titration and Diluting
iMV = iM2V2
MV = MV (concentrated = diluted)
oxidation vs reduction
loose electron vs gain electron
Calorimetry vs Bomb calorimetry
study of heat exchange, bomb has constant volume
Heat of Reaction
sum of heat of reaction of products minus sum of heat of reaction of reactants
bonds broken minus bonds formed
Hess’s Law
using multple reactions to figure out heat of reaction of one reaction