IB Chem Unit 7 (Kinetics)
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
When Atoms are Reacting
They must have collisions that lead to successful reactions.
Collision theory tells us the conditions required for successful collisions.
Collision Theory
The rate of a reaction is proportional to the rate of reactant collisions
The reactants must collide at a certain angle that allows contact between the atoms so that they bon.
The collision must happen with enough activation energy so that the electrons can rearrange and form new bonds and products.
Kinetics and Reaction Rates
Study of reaction rates
Change in concentration per unit time
NOT how long it takes for a reaction to finish.
Measured experimentally by…
Loss of reactants over time
Decreases with time
Production of products over time
Increases with time.
Changing Reaction Rates affects:
Temperature
Concentration
Surface Area
Based on effective collisions
Sufficient Energy
Activation energy
Proper Orientation
Correct angle.
Rate of Reaction on Temperature and Concentration
Temperature
As the temperature increases, particles move faster, increases more collisions, which leads to faster rate of reaction.
Concentration
Amount of solute dissolved in solvent
Increased concentration = particles closer = more collisions, = faster rate of reaction.
Rate of Reaction on Surface Area
Increased surface area of solid reactants means increasing more collisions, which leads to faster rate of reaction
Rate of Reaction on Catalysts
Catalyst increases the rate of reaction by lowering the minimum activation energy required to start a reaction to form products.

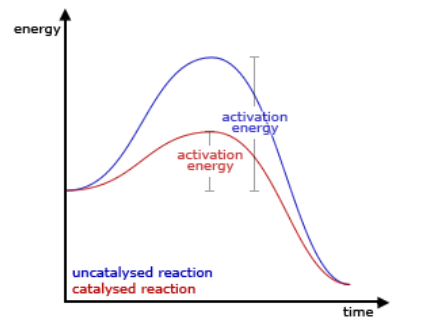
Catalyst need to be:
chemically unchanged at the end of the reaction (regenerated)
needs to change the mechanism of reaction
Decrease activation energy
Makes collisions more effective
Exothermic Reactions
Exothermic reactions release energy/heat to surroundings
Surroundings get warmer (feels hot to you)
ΔH < 0 (negative)
Products are more stable than reactants
Bonds made are more stable than bonds broken
Products have less energy than reactants
CH4 + 2O2 (g) 🡪 CO2 (g) + 2H2O (l) + energy
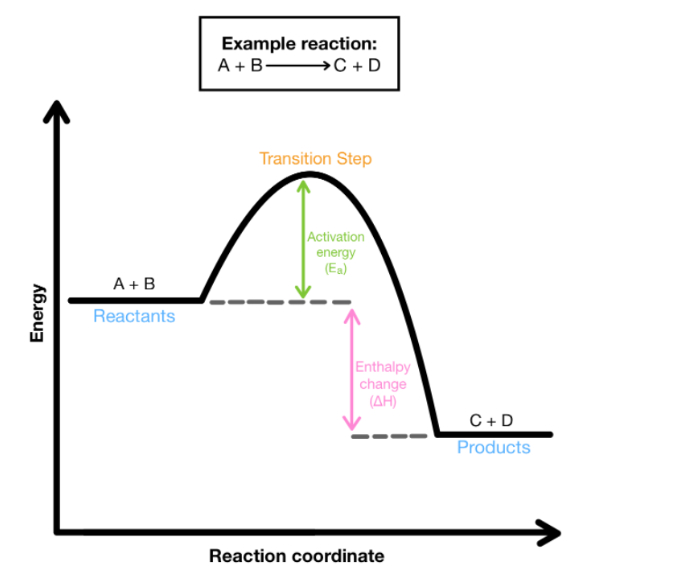
(exothermic) Enthalpy of products is:
Enthalpy of products is less than reactants.
Endothermic Reactions
Endothermic reactions absorb energy/heat from surrounding
Surroundings get colder (feels cold to you)
H > 0 (positive)
Bonds made are less stable than bonds broken
Products have more energy than reactants
NaHCO3 + HCl + energy 🡪 NaCl + CO2 + H2O
(Endothermic) Enthalpy of products is:
Enthalpy of products is greater than reactants
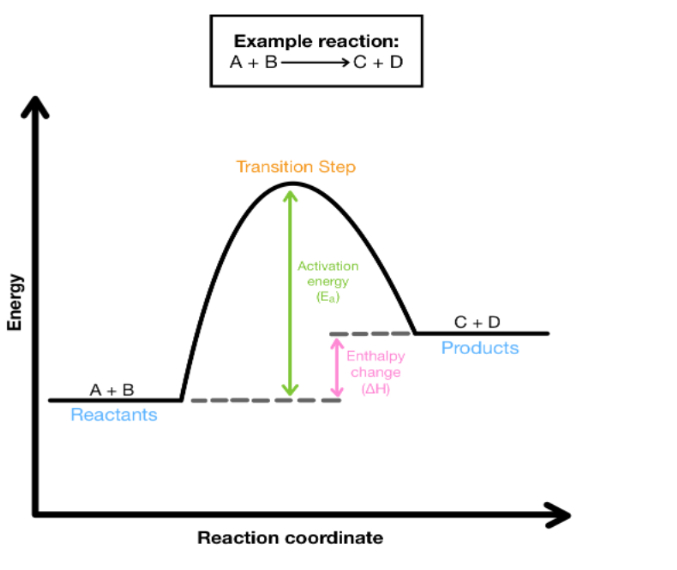
Reaction Pathways
Both diagrams start with an absorption of energy (activation energy)
Activation energy is the energy it takes to break existing bonds
Breaking bonds absorbs energy
Both diagrams end with a release of energy
Making new bonds releases energy
Chemical Reactions & Energy
Exothermic Reactions
Bonds in the products are stronger than bonds in the reactants
Endothermic Reactions
Bonds in the reactants are stronger than bonds in the products