Half life and Shelf life
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
12 Terms
reaction rate represents
the decomposition of the drug
Reaction rate refers to the rate at which a drug undergoes a chemical transformation, such as decomposition, hydrolysis, oxidation, or degradation over time.
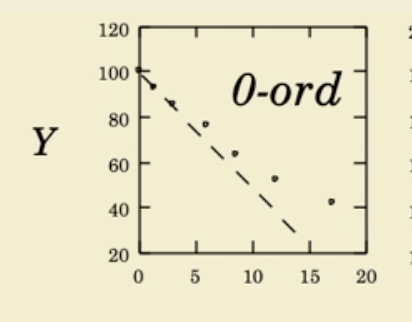
Zero order linear plot
X axis and Y axis
what is zero order and its half life
Concentration over time
the drug degrades at a constant rate regardless of how much remains. This means the drug is lost in equal amounts per unit time, and the half-life changes depending on the starting amount.
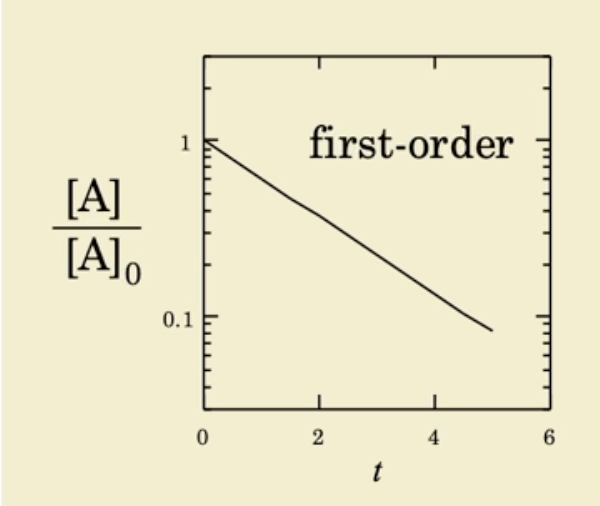
First order linear plot
what is zero order and its half life
Log concentration over time
first-order kinetics represent a reaction where the rate depends on the concentration of the drug. As the concentration decreases, the rate slows down — but the half-life remains constant because the drug always degrades by a fixed percentage per unit time.
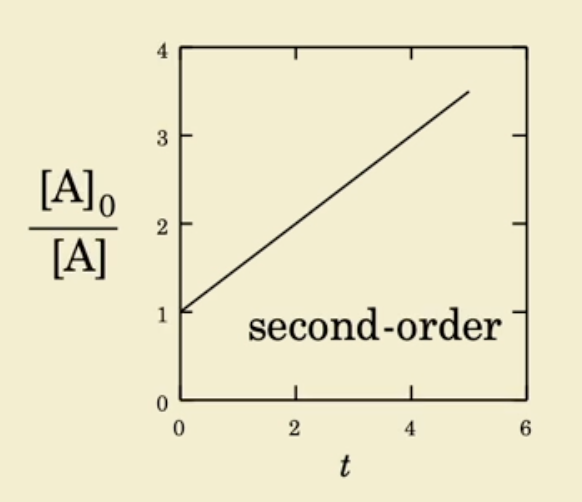
Second order linear plot
1/Concentration (1/[A]) over time
Best fit data based on previous order shows what?
best reaction rate law order
In what law does half life is constant?
first law
Does half-life stay the same if the degradation of aspirin in solution is first order?
yes because the rate of degradation depends on the concentration of the drug
Does half-life stay the same if the degradation of aspirin in suspension (= too much drug molecule in the solvent can handle) ?

The half-life changes when a drug is in suspension because the degradation follows zero-order kinetics. In a suspension, the drug is not fully dissolved, and as the dissolved portion degrades, more solid drug continues to dissolve to maintain a constant concentration at the saturation level. This leads to a constant degradation rate, and the half-life depends on the initial drug amount, so it is not constant.
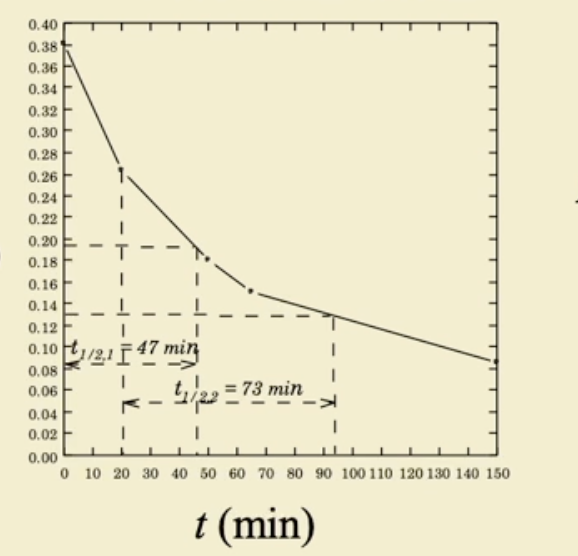
what happens to half life of 2nd law
half life decreases as the initial concentration increases
half life increases as the concentration decreases
explain aspirin in solution vs suspension
what is suspension?
suspension means the concentration of drug in solution is saturated at the solubility limit
When the concentration of aspirin exceeds the solubility limit of the solvent, a suspension forms. In this state, there is undissolved aspirin present. As the dissolved aspirin degrades (e.g., by hydrolysis), more solid aspirin dissolves to replenish it, maintaining a constant saturated concentration in solution. Because the dissolved concentration stays constant, the reaction rate also appears constant, which mimics zero-order kinetics — the drug degrades at a constant rate regardless of how much remains.
However, once all the excess solid aspirin is gone and the system becomes an unsaturated solution, the concentration of aspirin starts to decrease over time, and the degradation now follows first-order kinetics, where the half-life becomes constant and the rate depends on the drug’s remaining concentration.
shelf life affected by temperature is expressed using
Arrhenius equation

explain variable
A:
Ea:
range
R:
1.973cal/mol = kcal/mol
decrease in 10 degree temperature gives ___
frequency of molecule to achieve activation energy
activation energy = threshold energy to go forward or product side
10,000cal/mol ~ 30,000cal/mol = 10kcal/mol=30kcal
1.973×10^-3
reduce reactivity by 2-3 times