Exam2
1/95
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
96 Terms
enzyme converting ATP to cAMP
adenylyl cyclase
small hydrophilic molecule
cAMP
enzyme that breaks down cAMP into AMP through a hydrolysis reaction
cAMP phosphodiesterase
serine/threonine kinase regulates metabolic processes in cells and dependent on levels of cAMP in cell
cAMP dependent protein kinase
Binding of a signaling molecule GPCR on the cell membrane
Step 1 in cAMP signaling pathway
Activation of a G protein that stimulates adenylyl cyclase
Step 2 of cAMP signaling pathway
Leading to production of cAMP from ATP
Step 3 of cAMP signaling pathway
Activation of protein kinase A
Step 4 of cAMP signaling pathway
PKA phosphorylates target proteins to elicit cellular responses
Steps 5 of cAMP signaling pathway
Receptor, Activates cAMP and PKC, increases HR, stimulates energy production
Gs
Inhibitory, inhibits several signaling adenylyl cyclase, decrease heart rate, inhibits neurotransmitter release
Gi
the Gq protein pathway activates PLC, leading to the breakdown of PIP2 into IP3 and DAG. These products significantly affect cellular behavior by increasing intracellular calcium levels and activating PKC, ultimately resulting in a wide range of physiological responses such as contraction, secretion, growth, and survival.
gq system of g proteins leads to the activation of phospholipase c, the breakdown of pip2. Products of pip2 breakdown affect cellular behavior
as a response to hormones and transmitters, by a net influx of calcium ions into the cell
Ways in which intracellular Ca2+ concentration can increase
A rise in intracellular calcium concentration leads to binding of calcium ions to calmodulin, which binds and activates CaMKI I. Upon activation, this enzyme has the ability to autophosphorylate, a process that confers calcium-independent activity upon the kinase and greatly increases its affinity for calmodulin
an increase in cytosolic Ca2+ that alter cellular function through the activity of calmodulin-dependent protein kinases
Diffusion
Enzymatic degradation
Reuptake
Endocytosis
Competitive inhibition
Conformational changes
Ways that first messengers can be unbound from the receptor
Sodium
major positive ions extracellular
Chloride
Major negative ions extracellular
Potassium
Major positive ions intracellular
Phosphorous
Major negative ions intracellular
Opposite charges, such as a positive charge and a negative charge
Attractive
Like charges, two negative charges or two positive charges, will repel each other.
Repulsive
separated electrical charges of opposite sign have the potential to do work if they are allowed to come together
Electrical potential
Determined by the difference in the amount of charge between two points
Potential Difference
pull the sodium and chlorine ions apart so they are floating freely. These ions are what carry electricity through water, as these charged particles can freely move.
Why is water a good conductor of electricity
the electrical potential difference across a plasma membrane of an unstimulated cell
resting membrane potential
K+ that leaks from the inside of the cell to the outside via leak K+ channels and generates a negative charge in the inside of the membrane vs the outside
Why resting membrane potential exists
when the ion channel opens, the ion moves down its concentration gradient from high to low, in this case for K+ from the inside (intracellular region) to the outside (extracellular region).
Ion distribution across a neuronal membrane
potassium
Ion contribute most to the resting membrane potential
maintenance of electrochemical gradients across cell membranes by transporting three Na+ out of and two K+ into cells.
role of the Na+/K+-ATPase in ion distribution across the axonal membrane
determines the direction that ions will flow through an open ion channel
Electrochemical Gradient
The electrical potential difference across the cell membrane that exactly balances the concentration gradient for an ion
equilibrium potential
generates a negative charge in the inside of the membrane vs the outside.
How Na+ leak channels and K+ leak channels contribute to generation of the resting membrane potential
helps to maintain osmotic equilibrium and membrane potential in cells. The sodium and potassium move against the concentration gradients. The Na+ K+-ATPase pump maintains the gradient of a higher concentration of sodium extracellularly and a higher level of potassium intracellularly.
Role of the Na+/K+-ATPase in MAINTAINING the resting membrane potential despite the presence of leak channels
action potential in a presynaptic neuron increases the probability of an action potential occurring in a postsynaptic cell.
Excitatory Synapse
prevents hyperexcitability by providing activity-dependent inhibition.
Inhibitory Synapse
involves multiple neurons or neuronal cell types providing input onto a common postsynaptic partner,
Convergence
entails the distribution of synapses from an individual neuron onto multiple postsynaptic partners or partner types.
Divergence
the nerve impulse is transmitted chemically via neurotransmitters
chemical synapse
nerve impulse is transmitted electrically via channel proteins.
electrical synapse
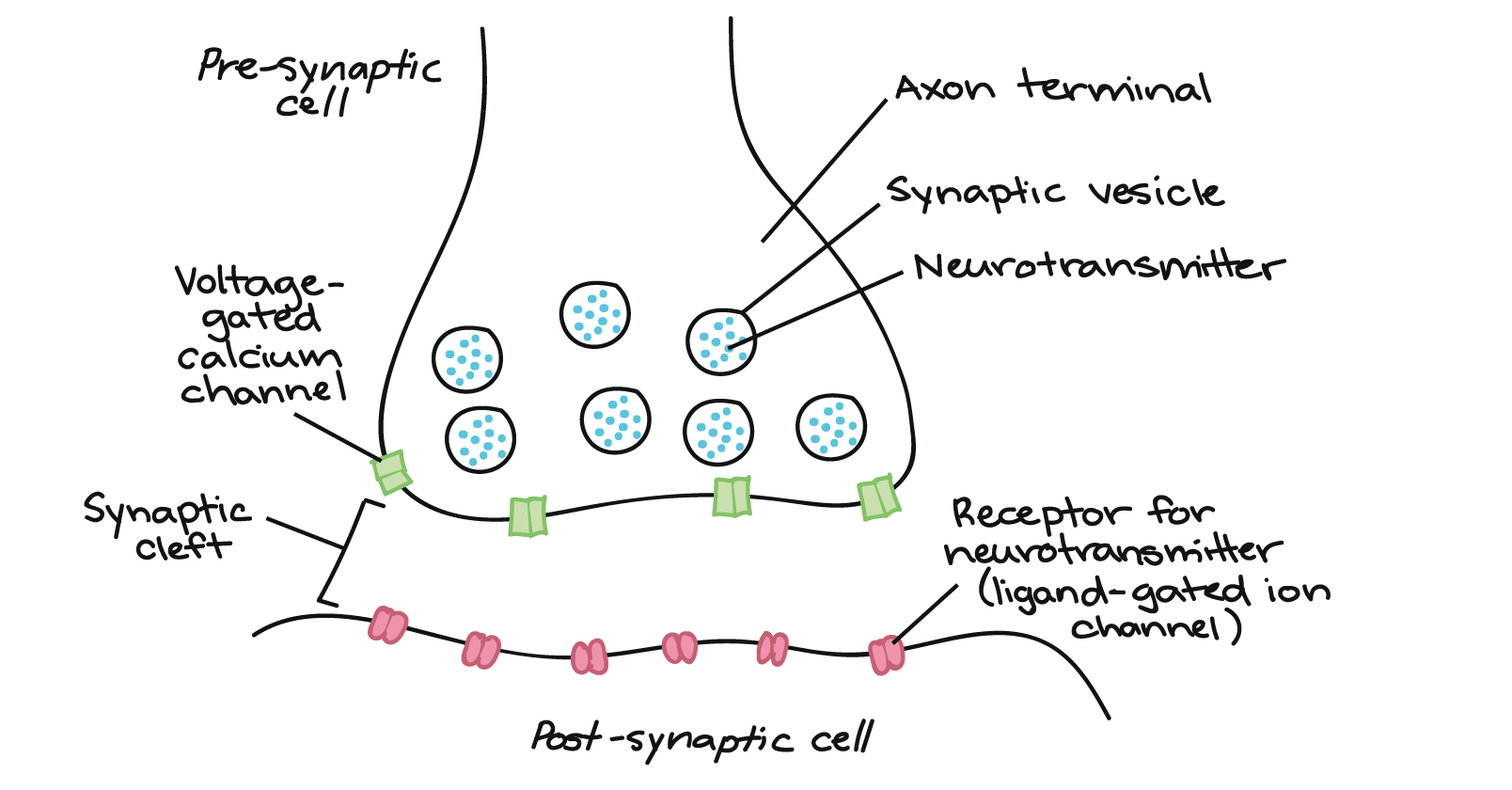
chemical synapse
Action Potential Arrival: The action potential travels down the axon of the presynaptic neuron and reaches the axon terminal.
Calcium Influx: The depolarization caused by the action potential opens voltage-gated calcium (Ca²⁺) channels in the presynaptic membrane. Calcium ions flow into the neuron.
Neurotransmitter Release: The influx of calcium triggers synaptic vesicles filled with neurotransmitters to move toward and fuse with the presynaptic membrane, releasing their contents into the synaptic cleft through exocytosis.
Neurotransmitter Binding: The released neurotransmitters diffuse across the synaptic cleft and bind to specific receptors on the postsynaptic neuron's membrane.
Postsynaptic Potential Generation: The binding of neurotransmitters to receptors can cause ion channels to open or close, leading to changes in the postsynaptic neuron's membrane potential. This can result in excitatory postsynaptic potentials (EPSPs) or inhibitory postsynaptic potentials (IPSPs).
Integration of Signals: If the excitatory inputs are strong enough to depolarize the postsynaptic neuron to a threshold level, it will generate its own action potential.
Termination of Signal: The neurotransmitter's action is terminated through reuptake into the presynaptic neuron, enzymatic degradation, or diffusion away from the synaptic cleft.
Recycling of Components: The presynaptic neuron can recycle neurotransmitter molecules and vesicular components for future use.
Transmission of a action potential from pre to post synaptic neuron
These proteins facilitate the docking and fusion of synaptic vesicles with the presynaptic membrane, while these act as calcium sensors that trigger the final release of neurotransmitters in response to calcium influx. Together, these proteins are essential for efficient synaptic transmission and communication between neurons.
SNARE proteins and synaptopgamins in synaptic transmission
mediate fast synaptic responses by directly controlling ion flow
Ionotropic Receptor
activate intracellular signaling pathways, leading to slower and more diverse cellular effects
Metabotropic Receptor
ensures that neurotransmitter signals are brief and that neuronal communication remains flexible and responsive to changing conditions
Neurotransmitter binding to the receptors is transient
as the number of unbound neurotransmitters decreases, the number of neurotransmitters bound to receptors also decreases, leading to reduced postsynaptic signaling.
NT bound to receptors
reuptake by the presynaptic neuron, enzymatic degradation in the synaptic cleft, diffusion away from the cleft, and uptake by glial cells
unbound neurotransmitters removed from synaptic cleft
excitatory neurotransmitters promote depolarization of postsynaptic neurons, generating excitatory postsynaptic potentials (EPSPs) that increase the likelihood of action potential firing
effect of NT’s on excitatory post synaptic neurons
function to promote depolarization in postsynaptic neurons, increasing their likelihood of firing action potentials. Sodium (Na⁺) and calcium (Ca²⁺) ions flow into the postsynaptic neuron, leading to this depolarization. As graded potentials, EPSPs can vary in magnitude and can summate to determine the overall excitability of the neuron.
excitatory and post synaptic potentials and ions responsible for the direction of flow
inhibitory synapses and IPSPs function to reduce the excitability of postsynaptic neurons, making it less likely for them to fire action potentials. Chloride (Cl⁻) and potassium (K⁺) ions are primarily responsible for these inhibitory effects, with Cl⁻ flowing into the neuron and K⁺ flowing out. As graded potentials, IPSPs can vary in magnitude and interact with other postsynaptic potentials to modulate neuronal activity.
inhibitory synapses and post synaptic potentials and ions responsible for direction of flow
rapid succession of inputs from a single source to build a stronger signal
temporal summation
integrates signals from multiple sources simultaneously
spatial summation
postsynaptic neuron will fire an action potential or remain resting.
outcome of of spatial and temporal summative events
integration point for synaptic inputs, its high density of voltage-gated sodium channels, and its ability to determine whether the membrane potential reaches the threshold needed to initiate an action potential.
importance of axon hillock generating action potentials
high density of voltage-gated sodium channels, specific membrane properties, efficient integration of synaptic inputs, and its role in action potential generation.
threshold potential is lower at the axon hillock
responsible for fast communication between neurons
neurotransmitter
adjust and fine-tune that communication over a longer duration, influencing a broader range of neural activity
neuromodulator
Amino acids, biogenic amines, neuropeptides, purines, gasotransmitters, acetylcholine
classes of neurotransmitters and neuromodulators
involves the combination of acetyl-CoA and choline, catalyzed by choline acetyltransferase. The resulting ACh is then stored in synaptic vesicles until released into the synaptic cleft, where it can bind to receptors on the postsynaptic cell, facilitating neurotransmission.
acetylcholine synthesis
crucial role in terminating the action of acetylcholine in synapses, ensuring that signals are brief and well-regulated. AChE inhibitors increase the duration of ACh action, enhancing synaptic transmission but also carrying the risk of overstimulation and toxicity.
Function of acetylcholinesterase
ACh receptor that primarily mediate fast synaptic transmission and muscle contraction,
Nicotinic receptor
ACh receptor involved in slower, modulatory actions within the autonomic nervous system and the brain.
Muscarinic Receptor
Dopamine, Norepinephrine, Adrenaline
NT’s that are catecholamines
crucial for the degradation of monoamines, regulating their levels in the synapse. These inhibitors enhance neurotransmission by preventing the breakdown of these neurotransmitters, leading to increased synaptic levels, which can have therapeutic effects, particularly in treating depression.
Monoamine oxidase
Location: Blood vessels, CNS, smooth muscle, organs |
Mode of Action | GPCRs (Gq protein) for a-1; inhibitory for a-2 |
Subtypes | A-1, A-2 |
Effectors | Vasoconstriction, inhibition of neurotransmitter release (a-2) |
Alpha Adrenergic Receptor
Location | Heart, lungs, smooth muscle, adipose tissue |
Mode of Action | GPCRs (Gs protein) |
Subtypes | Beta-1, Beta-2, Beta-3 |
Effectors | Increased heart rate (beta-1), bronchodilation (beta-2), lipolysis (beta-3) |
Beta-adrenergic Receptor
crucial for modulating a variety of physiological functions in the nervous system, including pain regulation, mood modulation, and homeostasis. Examples include substance P, which is primarily associated with pain transmission, and endorphins, which help manage pain and promote feelings of pleasure.
neuropeptides
General Function: Prepares the body for "fight or flight" responses during stressful situations.
Effects:
Increases heart rate and blood pressure.
Dilates airways to improve breathing.
Inhibits digestive processes.
Mobilizes energy stores by stimulating glucose release from the liver.
Increases blood flow to skeletal muscles while reducing flow to non-essential organs.
Sympathetic Nervous System
General Function: Promotes "rest and digest" activities, conserving energy and maintaining homeostasis during calm situations.
Effects:
Decreases heart rate and blood pressure.
Constricts airways.
Stimulates digestive processes, including saliva production and peristalsis.
Promotes nutrient absorption and storage.
Increases blood flow to digestive organs.
Parasympathetic Nervous System
consists of preganglionic neurons originating from the CNS, autonomic ganglia where synapses occur, and postganglionic neurons that innervate target organs. The sympathetic division promotes widespread responses, while the parasympathetic division facilitates more localized control.
Anatomical organization of autonomic motor system
both divisions of the ANS utilize ACh in their preganglionic neurons, but they differ significantly in the neurotransmitters used by postganglionic neurons and the resulting physiological effects. But this division prepares the body for action, while the parasympathetic division promotes relaxation and recovery.
preganglionic and postganglionic neurons
contains only one neuron because it functions as a specialized structure that directly releases hormones into circulation, bypassing the need for an intermediate postganglionic neuron.
sympathetic pathway to the adrenal medulla
release of epinephrine and norepinephrine plays a critical role in the body’s response to stress, enabling rapid physiological adjustments to prepare for immediate physical demands. These hormones work synergistically to enhance alertness, energy availability, and physical performance during stressful situations.
chemicals released from adrenal medulla
key feature of the ANS that allows for intricate control of various bodily functions, enabling the body to respond appropriately to different situations. This interplay between the sympathetic and parasympathetic divisions ensures that organs can be activated or inhibited as needed, contributing to overall physiological balance and homeostasis.
Dual Innervation
key electrical change in cells that initiates processes like action potentials in neurons and contractions in muscle cells.
Depolarize
process of restoring a cell's resting membrane potential after depolarization.
Repolarize
cell's membrane potential becomes more negative than its resting state, making it less likely to fire an action potential.
Hyperpolarize
occurs when positively charged sodium ions enter the cell, causing the inside of the cell to become less negatively charged (more positive). This process is crucial for the generation of action potentials in neurons and muscle cells, enabling them to communicate and function effectively.
Depolarization of the membrane
stronger stimuli lead to larger graded potentials. This relationship allows cells to encode the intensity of stimuli, which is crucial for processes such as sensory perception and neuronal signaling.
relationship between strength of graded potential and strength of stimulus
diminishes over distance due to factors such as current leakage and the resistance of the cell membrane.
strength of a graded potential
through temporal and spatial mechanisms, significantly affects the strength of graded potentials. This process enhances the ability of neurons to respond to varying intensities and frequencies of input, ultimately influencing whether or not an action potential will be generated.
summation of multiple stimuli
Skeletal, Cardiac, Smooth, Neurons, Endocrine Cells, Sensory Cells are crucial for transmitting signals and facilitating responses to stimuli throughout the body.
Cells that have excitable membranes
involves a series of well-coordinated events: depolarization (Na⁺ influx), repolarization (K⁺ efflux), and often hyperpolarization. These rapid changes in membrane potential allow for the transmission of signals along neurons and muscle fibers, making action potentials fundamental to nervous system and muscle function.
Events occurring in an action potential
Local changes in membrane potential |
Magnitude | Varies with stimulus strength |
Duration | Longer-lasting (milliseconds to seconds) |
Propagation | Local, diminishes with distance |
Threshold Requirement | No threshold required |
Summation | Can summate (temporal and spatial) |
Types | Can be excitatory or inhibitory |
Graded Potentials
Rapid, all-or-nothing changes in membrane potential |
Magnitude | Fixed magnitude (same for each action potential) |
Duration | Short-lived (1-2 milliseconds) |
Propagation | Propagates without decrement |
Threshold Requirement | Requires reaching a specific threshold |
Summation | Does not summate; all-or-nothing response |
Types | Always excitatory |
Action Potentials
Respond to chemical signals, leading to localized changes in membrane potential.
Ligand-Gated Ion Channels in changing membrane voltage
Respond to changes in membrane voltage, crucial for the propagation of action potentials.
Voltage-Gated Ion Channels in changing membrane voltage
Respond to mechanical stimuli, allowing cells to detect and respond to physical changes in their environment.
Mechanically-Gated Ion Channels in changing membrane voltage
Depolarization: Initiated by the rapid opening of voltage-gated sodium channels, allowing Na⁺ influx.
Repolarization: Caused by the closing of sodium channels and the opening of voltage-gated potassium channels, leading to K⁺ efflux.
Afterhyperpolarization: Continued K⁺ outflow results in a temporary hyperpolarized state.
Return to Resting State: Sodium-potassium pumps help restore ion concentrations and stabilize the membrane potential.
Ion channels initiate and end of an action potential
membrane voltage level that must be achieved for an action potential to be initiated, marking a crucial point in the excitability of neurons and muscle cells. It underpins the all-or-nothing principle of action potentials, ensuring that a sufficient stimulus leads to a full-scale electrical response.
Threshold potential
asserts that once the threshold is reached, an action potential is generated with a consistent amplitude and duration.
All-or-none law of action potential
No action potential can occur, primarily due to inactivated Na⁺ channels; lasts for about 1-2 milliseconds.
Absolute Refractory Period
A stronger-than-normal stimulus can generate an action potential, influenced by the partial recovery of Na⁺ channels and ongoing K⁺ efflux; lasts for about 2-4 milliseconds.
Relative Refractory Period
Conduct action potentials through continuous conduction, with sequential depolarization along the entire length of the axon, resulting in slower signal transmission (1-10 m/s)
Action potentials in unmyelinated axons
Conduct action potentials through saltatory conduction, where the action potential jumps between Nodes of Ranvier, allowing for much faster signal transmission (up to 120 m/s).
Action potentials in myelinated axons
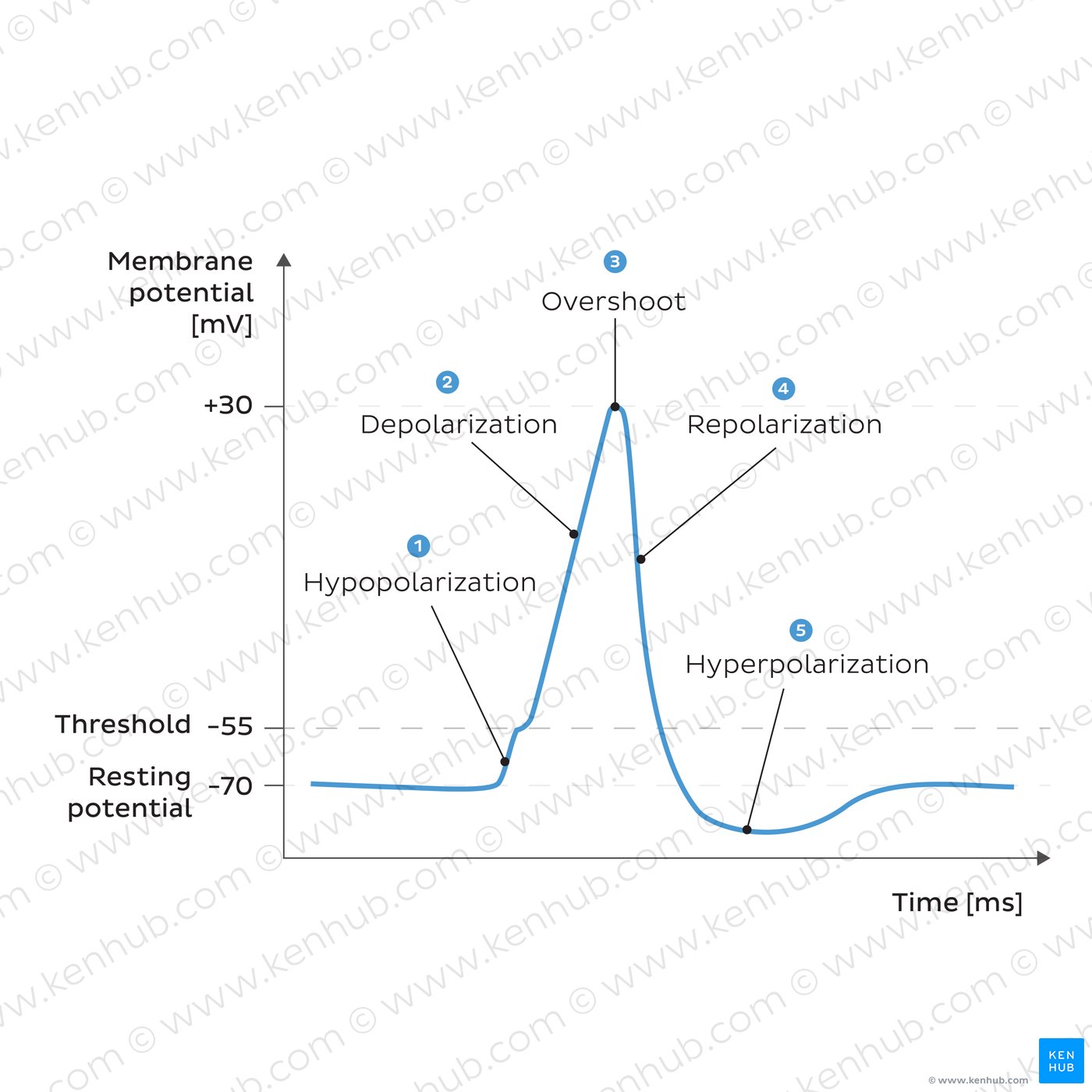
Action Potential