9. Chirality 3&4
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
What effect, if any, does more than 1 chiral carbon have on their properties?
just answer this :The more asymmetric carbons present,
- The more asymmetric carbons present, the more stereoisomers are possible.
2 to the power of n (n =number of chiral carbons )
=detmine how many steriomers are persent
What is the meso form?
the (r,s) or (s,r) enatiomers is called the meso form
• Meso compounds have two or more asymmetric carbons and a plane of symmetry.
meso compounds are achiral
How do you identify stereoisomers with 2 chiral carbons?
non-superimposable mirror images
Non super impossible, not mirror images of same structural formula
Superimposable, has mirror plane
=enantiomers
> diastereoisomers
> meso compound
do disterioerms have the same chemical propeties +phsycial propeeties
> mostly the same chemical reactivity (because they have the same functional groups)
> different physical properties
How can you separate enantiomers?
By resolution
By chiral chromatography
What is resolution?
The separation of enantiomers from either a racemic mixture or enantiomerically enriched mixture
What is chiral chromatography?
where a racemic solution is passed over a chiral column. giving a rapid and reversible disterootropic interaction.
What enables diastereoisomers to separate?
The different physical characteristics
Explain how you could separate 2 diastereoisomers by their solubility in solvents?
Diastereoisomers have different physical properties including solubility in different solvents.
The different solubility in solvents can be used to separate the diastereoisomers.
Use a solvent in which one diastereoisomer is soluble and the other is less soluble.
The less soluble isomer will crystallise and can be filtered.
The other diastereoisomer can then be obtained by evaporating the solvent
How can we recover enantiomers from seperated disterosimeric esters?
use aquous acid
Consequences of chirality
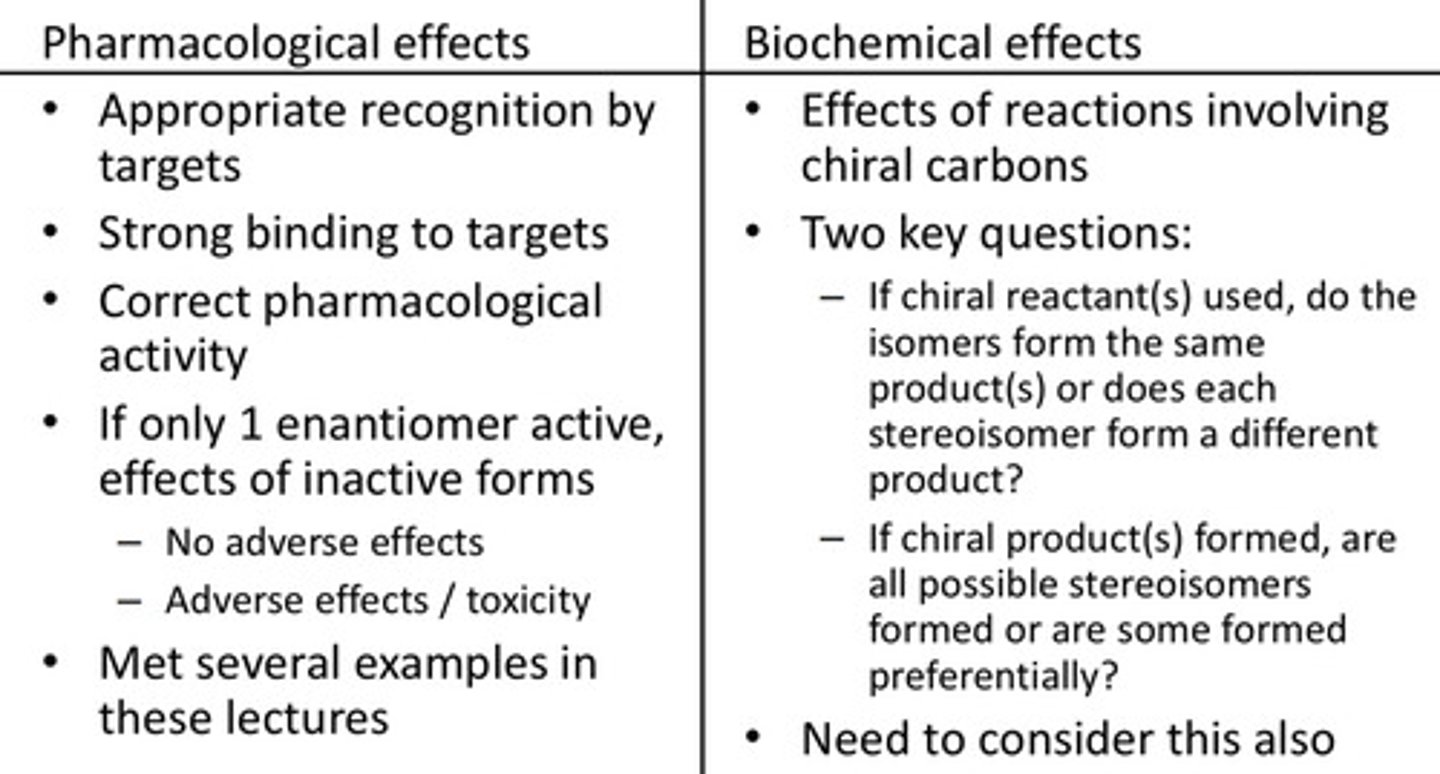
Reactions with chiral molecules: what is a stereoselective reaction?
Preferential formation of one stereoisomer over another
(Expensive method)
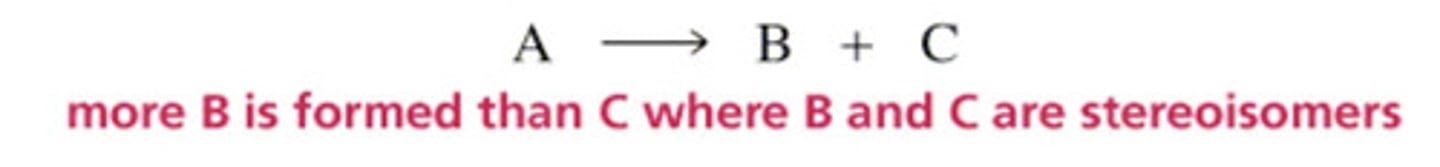
Reactions with chiral molecules: what is a stereospecific reaction?
Each stereoisomeric reactant produces a different stereoisomeric product or a different set of products
Stereoselective vs stereospecific

What makes stereo-selectively possible in reactions?
- It is possible due to mechanism
- If chiral reactant and/or product, mechanism:
> can show no stereoselectivity (resulting in racemisation)
> or can show stereoselectivity
What are stereochemical outcomes important in?
- Metabolism of drugs
- Drug action at receptors
- Toxicity through undesirable activity of stereoisomers
What is a concerted reaction?
- Where bonds are breaking and new bonds are forming at exactly the same time
What is a non-concerted reaction?
- Where some bonds breaks and then after some new bonds form (doesn't happen at the same time).
Concerted vs Non-concerted reactions

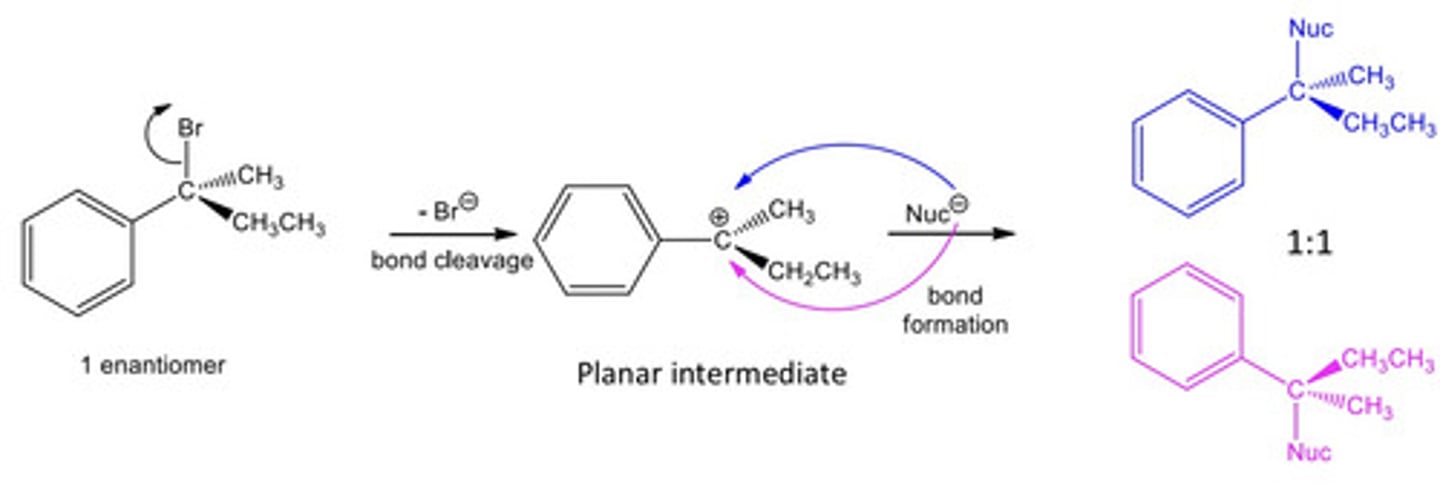
Key points about non-concerted reaction: SN1
- These reactions always occur at sp3 hybridised carbons
- Always more than 1 step
1. 1st step: polarised bond between C and heteroatom (Br) breaks
2. Intermediation carbocation is formed: planar
3. Reactant forms new bond to C, and could do this by attacking planar intermediate from either above or below the plane equally easily.
4. 3D tetrahedral carbon reformed with a 1:1 mix of enantiomers (a pair of enantiomers)
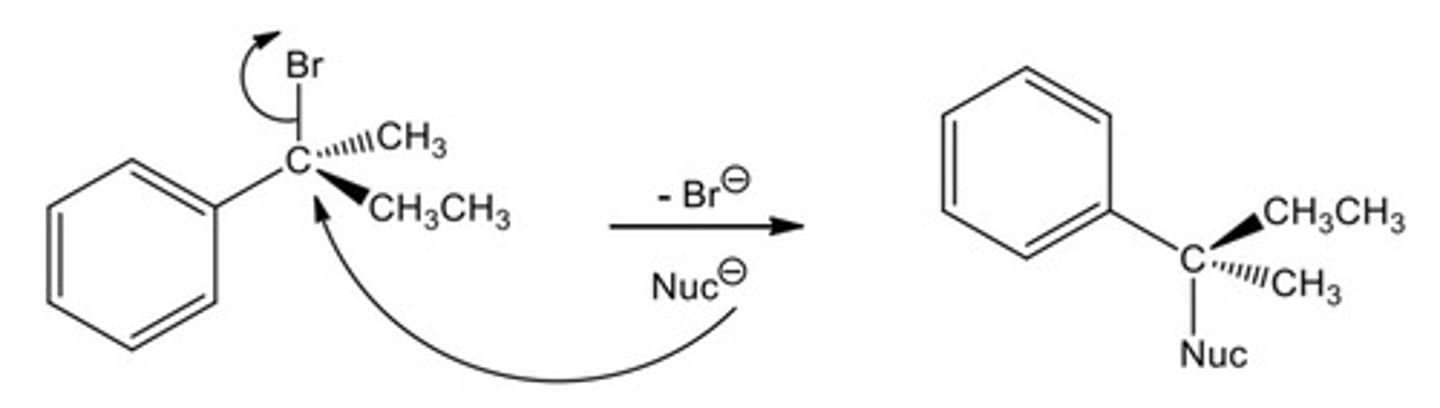
Key points about concerted reaction: SN2
- These reactions occur at sp3 hybridised carbons
1. Polarised bond between C and heteroatom (Br) causes slight negative charge (s+) on C making it electrophilic
2. Nucleophile attacks electrophilic C s+, breaking existing polarised C-Br bond as new bond is made - one step reaction
3. No intermediate is formed
4. Single product is formed (unlike SN1 which produces a pair of enantiomers)
- SN2 is a Walden inversion (the chiral centre is flipped); think of it as an umbrella that get flipped inside out on a windy day.
how do you seperate 2 enatiomers? long
Enantiomers have the same physical properties making them more difficult to separate.
You could use chiral chromatography where a racemic mixture is passed over a chiral column.
Another method is to make a salt using another chiral molecule (either an acid or a base).
This creates 2 diastereoisomers, which then have different physical properties and can be separated more readily.