Collision theory and Boltzmann Distribution
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
What is the formula and unit for rate of reaction?
change in conc./time(s)
unit = moldm-3 s-1
What are the 5 factors affecting rate of reaction?
Temperature
Pressure (only in gas)
Concentration
Catalyst
Surface Area
What makes collisions successful?
The energy needs to be below or above activation energy
The particle must be in the right orientation (right parts of particles must collide)
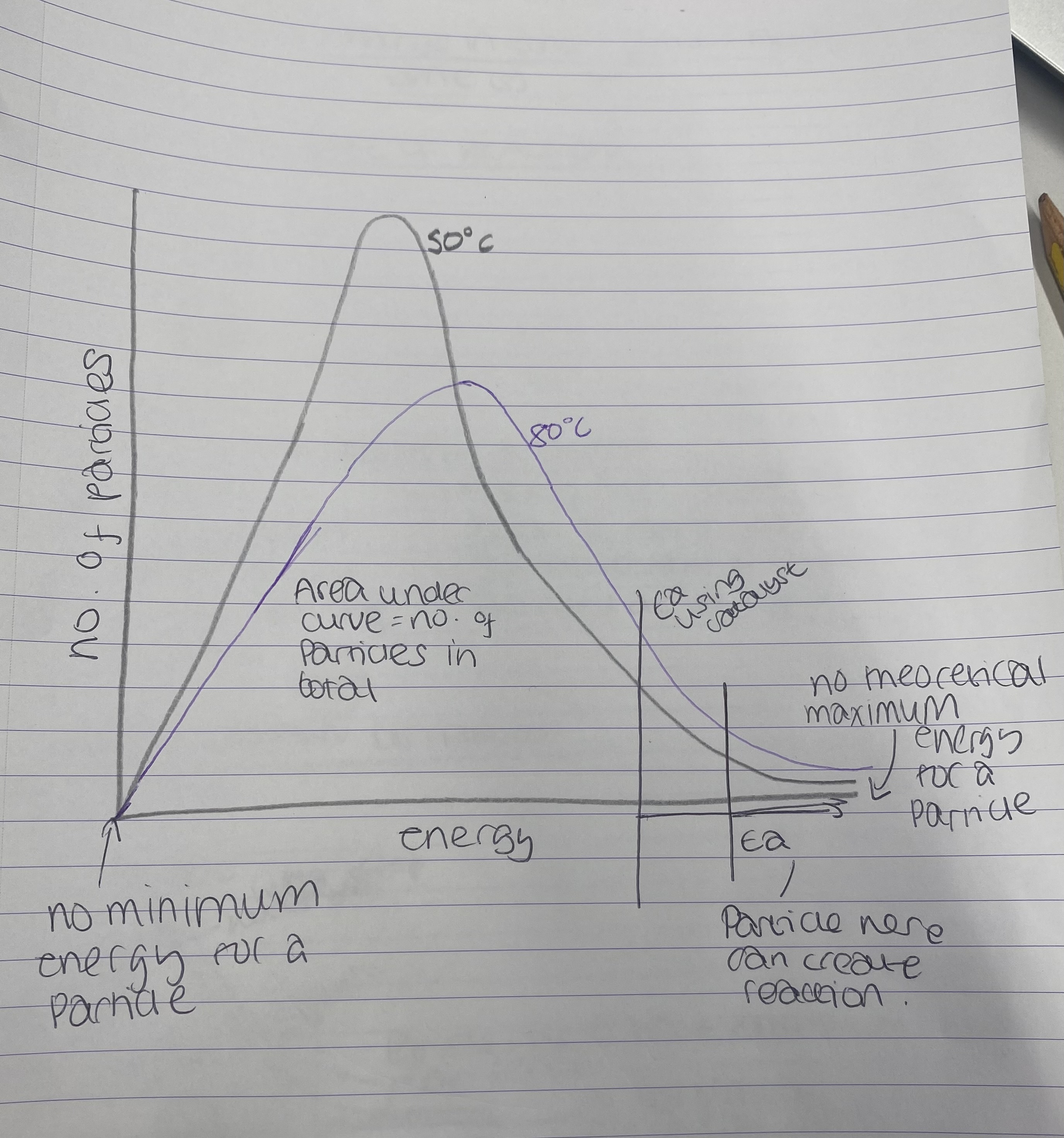
What does Boltzmann distribution look like?
.
How does concentration affect rate of reaction?
The higher the conc. the faster the rate of reaction. Those causes more particles in a given volume, the particles will then have energy above Ea causing more frequent collision. Therefore creating successful collisions
How does pressure affect the rate of reaction?
The higher the pressure, the faster the rate of reaction. This creates less space for the particles, causing more frequent collisions. Creating more successful collisions
How do catalysts affect the rate of reactions?
Catalysts speed up reactions by offering an alternative pathway with a lower Ea. The particles will have energy above Ea. Creating more successful collisions.
How does temperature affect the rate of reaction?
Temperature gives the particles more energy. This causes the particles to move faster (increase in kinetic energy). Causing more frequent collision above Ea. Creating successful collisions.