Bonding, structure, and the properties of matter
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
What are the three types of strong chemical bonds
ionic, covalent and metallic
What is ionic bonding
Ionic bonding is the electrostatic attraction between positive and negative ions.
How are ionic compounds held together?
They are held together in a giant lattice.
It’s a regular structure that extends in all directions in a substance.
Electrostatic attraction between positive and negative ions holds the structure together
State properties of ionic substances
High melting and boiling point (strong electrostatic forces between oppositely charged ions).
Do not conduct electricity when solid (ions in fixed positions).
Conduct when molten or dissolved in water – ions are free to move.
Give 5 examples of positive ions and 5 examples of negative ions.
What is important when working out a formula of an ionic compound?
Positive ions: Na⁺, Mg²⁺, Al³⁺, Ca²⁺, Rb⁺
Negative ions: Cl⁻, Br⁻, SO₄²⁻, NO₃⁻, OH⁻ (chloride, bromide, sulfate, nitrate, hydroxide)
Ionic compounds are electrically neutral (positive and negative charges balance).
How are ionic compounds formed? Explain in terms of MgO case.
Reaction of a metal with a non-metal.
Electron transfer occurs – metal gives away outer shell electrons to non-metal.
Mg (Group II) has 2 outer shell electrons.
O (Group VI) can accept 2 electrons.
Mg becomes Mg²⁺ and O becomes O²⁻ (oxide).
What is a covalent bond?
A covalent bond is a shared pair of electrons between two atoms.
Describe the structure and properties of simple molecular covalent substances
Do not conduct electricity (no ions).
Small molecules.
Weak intermolecular forces.
Low melting and boiling points.
How do intermolecular forces change as the mass/size of the molecule increases?
They increase.
This causes melting/boiling points to increase as well (more energy needed).
What are polymers?
Polymers are very large molecules (hundreds or thousands of atoms) linked by covalent bonds.
What are thermosoftening polymers?
melt/soften when heated because weak intermolecular forces between chains can be overcome
What are giant covalent substances? Give examples
Solids, atoms covalently bonded together in a giant lattice.
High melting/boiling points (strong covalent bonds).
Mostly don’t conduct electricity (no delocalised electrons).
Examples: diamond, graphite, silicon dioxide.
Describe and explain the properties of allotropes of carbon
Diamond:
Four strong covalent bonds per carbon.
Very hard.
Very high melting point.
Does not conduct (no delocalised electrons).
Graphite:
Three covalent bonds per carbon.
Layers of hexagonal rings.
High melting point.
Layers slide (weak intermolecular forces) → soft, lubricant.
Conducts heat and electricity (one delocalised electron per carbon).
Fullerenes:
Hollow shaped molecules of hexagonal rings (may include 5- or 7-membered rings).
C₆₀ = Buckminsterfullerene (spherical).
Nanotubes:
Cylindrical fullerenes with high length-to-diameter ratio.
High tensile strength.
Conductive (delocalised electrons).
Graphene: single layer of graphite.
What is metallic bonding?
Forces of attraction between delocalised electrons and nuclei of metal ions.
Describe properties of metals
High melting/boiling points (strong attraction).
Good conductors of heat and electricity (delocalised electrons).
Malleable/soft (layers of atoms slide over each other).
What are alloys? Why are they harder than pure metals?
Alloys = mixture of metal with other elements.
Different atom sizes distort layers, so they cannot slide → harder than pure metals.
What are the three states of matter?
Solid, liquid, gas.
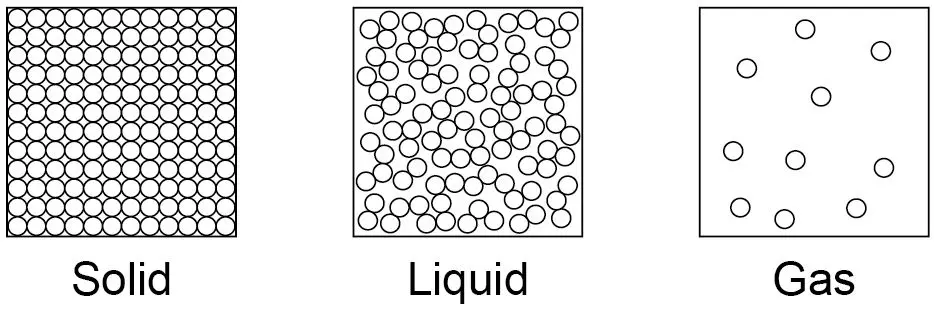
what take place at the melting point and what take place at the boiling point.
Melting and freezing take place at the melting point, boiling and condensing take place at the boiling point.
What does the amount of energy needed to change state depend on?
The strength of the forces between the particles.
Depends on bonding and structure.
Stronger forces = higher melting and boiling points.
A pure substance will melt or boil at…?
A fixed temperature.
A mixture will melt over a range of temperatures.
What is nanoscience?
Science that studies particles that are 1 - 100nm in size
State the uses of nanoparticles
- Medicine (drug delivery systems)
- Electronics
- Deodorants
- Sun creams (better skin coverage and more effective protection against cell damage)
What are fine and coarse particles?
- Fine particles (soot), 100-2500 nm diameter
- Coarse particles (dust), 2500-105 nm diameter
Why do nanoparticles have properties different from those for the same materials in bulk?
High surface area to volume ratio