7/8) Activity of aqueous species & Solubility and saturation states
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
37 Terms
what are the standard states defined as?
298.15K and 1 bar
gas: 1 atm ideal gas
liquid: pure liquid
solid: pure solid (not a solid solution)
solvent (liquid or solid): pure solvent
solute: 1 mol/kg (rarely do we have 1 mol of anything)
these are ideal conditions
when y=1 and x=1
what are the reference states defined as?
where the activity coefficient is equal to unity (unity=1) (y=1)
liquid: pure liquid
solid: pure solid
solvent (liquid or solid): pure solvent
solute: infinitely dilute 1m solution (as if activity coefficient is 0
the solute reference state is by definition impossible, you can’t be infinitely dilute (have nothing in solution) and also have 1 molal. it’s done to make the math easier (divide by 1 instead of nanomolals)
what can cause activity to not equal concentration?
electrostatic shielding
aqueous complexes
what does electrostatic shielding depend on? how is it accounted for?
ionic strength of solution and the size/charge of the ion itself
tiny and high charge = significant interactions with other ions
accounted for by activity coefficients
what do aqueous complexes depend on? how are they accounted for?
strength of the complexes and concentration of the complexing agent
if you have a ton of sodium and chlorine in solution, more of them will be in complexes just because there is a larger concentration of them in solution
accounted for by speciation
explain distance of closest approach
how close ions can get to other ions
caused by water molecules orienting themselves around a charged ion due to their polar nature. and much more water than ions in a solution (96%)
causes decrease in effective charge and increase in apparent size (hydrated radius)
higher charge _____?
higher charge attracts more solvent
increased hydrated radius ___?
reduces ability of ions to interact with each other, lowers the activity of an ion in solution
results in less solvent attracted (charge is more diffuse)
what is activity in simple terms
what proportion of the ion is actually doing reactions
what is activity coefficient
a translator between activity and concentration
thermodynamics speaks in activities, but we can only measure concentrations
varies between 1 and 0
as concentration decreases, the solution becomes more ideal (because we defined the reference solution to be infinitely dilute
what is ionic strength?
equation
what most influences ionic strength?
measure of total charge in a solution
I = ½ sum of all molar concentrations*charge²
charge really really affects ionic strength
details of variables in B-dot (DH) equation
Ay and By are calculated from physical constants (to do with water)
Anaught: distance of closest approach
Bdot: temperature dependent correction factor to allow debye-huckel to work in solutions with elevated ionic strength
DH works better at higher temps
explain the matlab example of different charges
higher charges decrease in activity much quicker than lower charges
if Anaught (distance of closest approach) is smaller, then H+ has lots of water guarding it, so other ions can’t attack because it’s guarded. and K+ has less water, so other ions can attack more readily, activity goes down quicker.
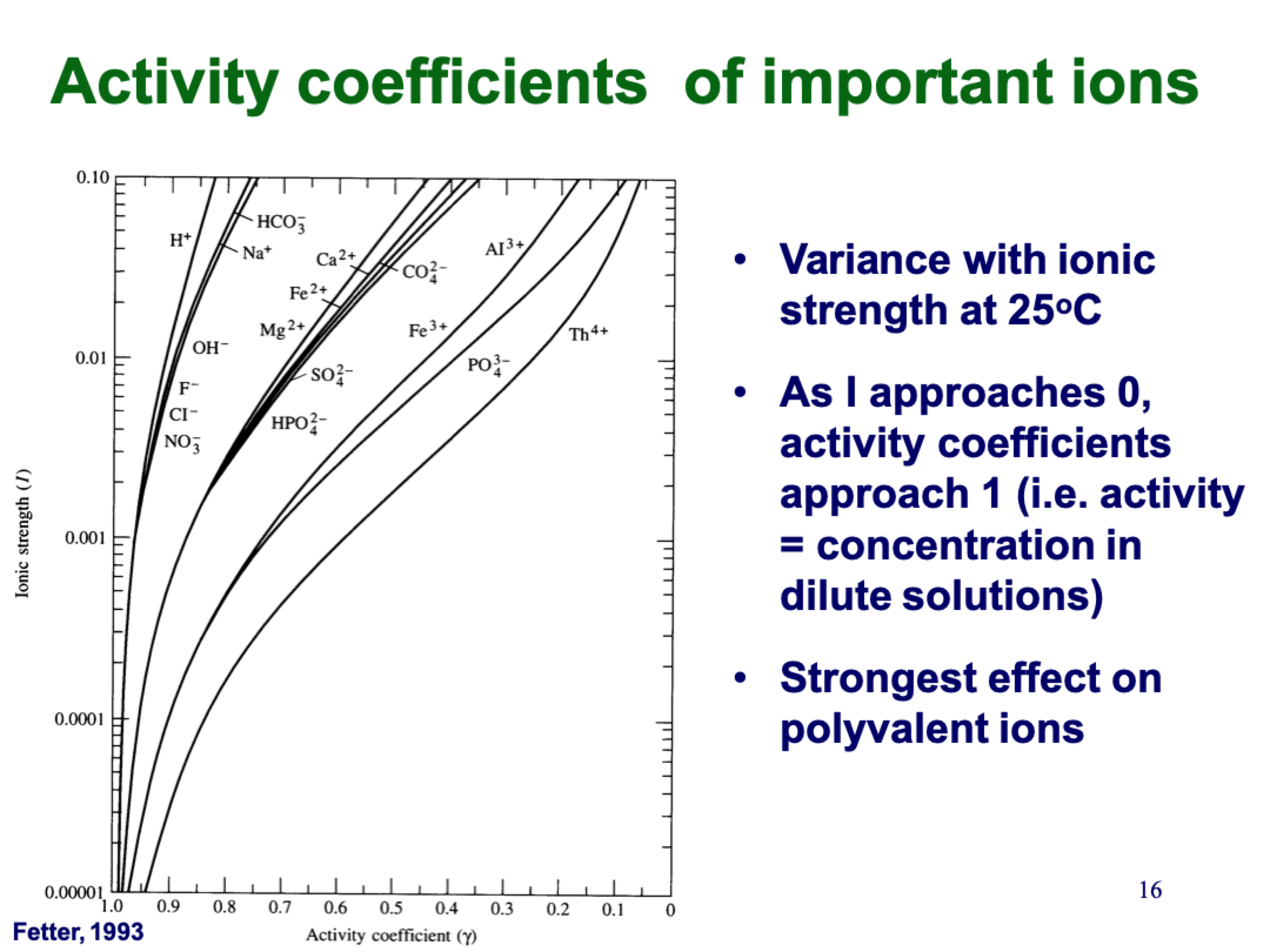
explain this
charge has a bigger different than size, so lines cluster in the charges (pos/neg doesn’t matter it’s squared)
strongest effect on polyvalent ions (in different clusters)
how does DH compare to measured values?
good job on any temperature up to 3 molal solutions, and predicts act coeffs up to IS=1
does not predict 6 molal below 150°C
works better in concentrated solutions at high temp
what are the activity coefficients of dissolved gases like?
they increase as ionic strength increases, starting at >1
none of them have act coeff of 1 at high IS
all neutral species behave this way
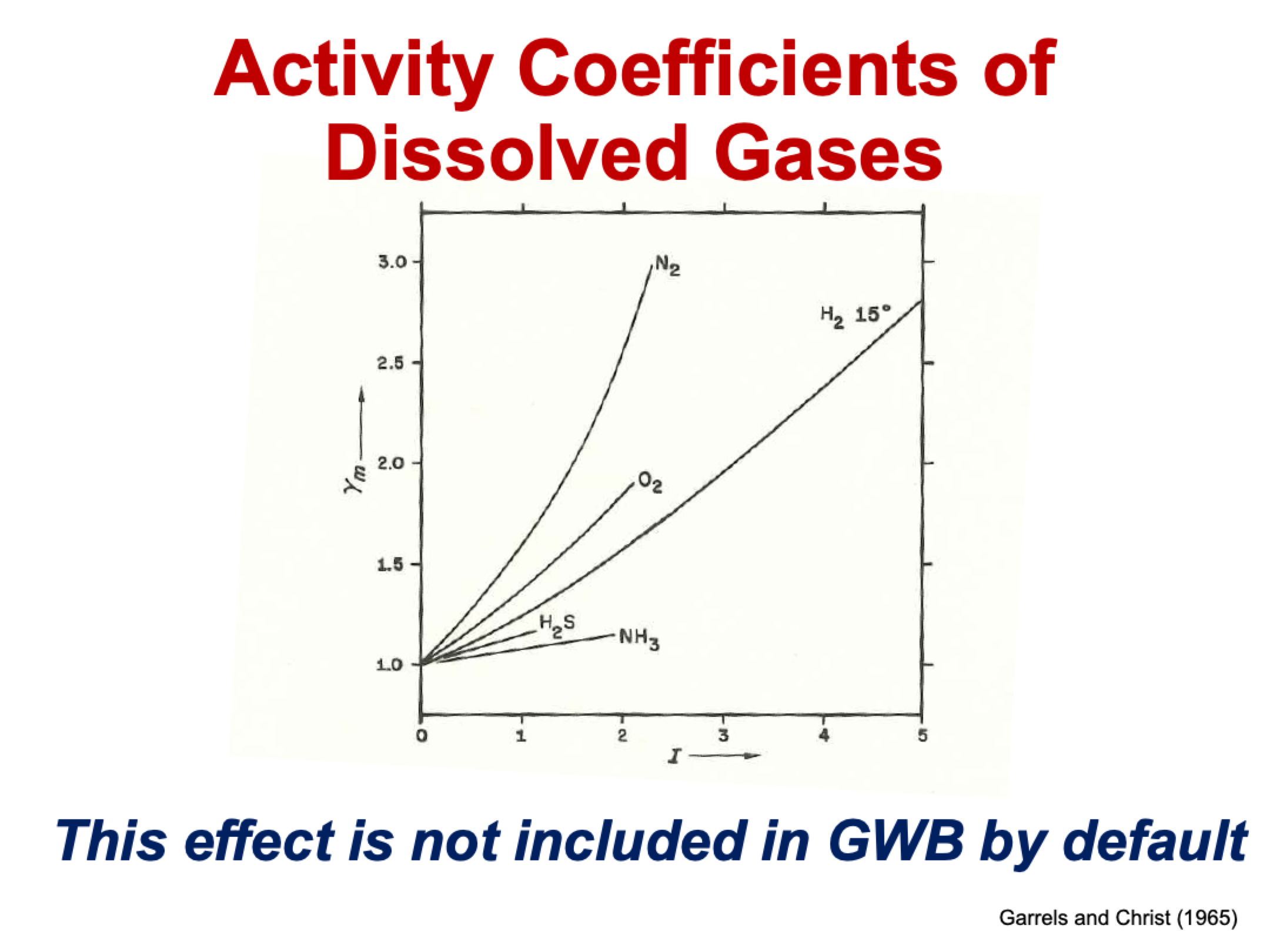
what is “salting out”
gamma(CO2(aq)) increases with increasing IS
KH = gammaCO2(aq) * m CO2(aq) / fCO2
KH and fCO2 are still fixed, so mCO2(aq) must be decreasing
as we add salt, CO2 goes out of solution
how to calculate saturation index
sat ind: Q/K
Q: (gammaCa2+ * mCa2+) * (gammaCO32- * mCO32-) / calcite
K: {Ca2+}*{CO32-} / calcite
ionically bound minerals such as ___, and sulphate minerals such as ____, have simple solubility relationships which are (almost) ____
halite
gypsum
independent of pH
solubility of halite is basically the same whether pH is 2 or 10
solubility in simple terms
the concentration of the species in solution
the amount of a mineral that can be dissolved in water
the sum of all relevant species
halite solubility simplified
Ksp = {Na+}*{Cl-} / {NaCl(s)} = {Na+}*{Cl-} = {Na+}² = {Cl-}²
simplify first because {NaCl(s)} = 1 and then {Na+} = {Cl-} when we dissolve halite in pure water
explain solubility product constant Ksp
when multiplied together, activities of Na+ and Cl- give a constant value solubility product constant, Ksp
one of the ions can be really high an dhte other very low, all we know is that the product gives the Ksp value, for halite Ksp=36
log Ksp = log{Na+} + log{Cl-} = 1.55
what is importance of Rimstidt data
14 year experiments to determine quartz solubility
databases are systematically offset now
we know ∆G because we know K (∆G = -RTlnk)
explain the solubility of quartz as it relates to pH
not pH dependent below pH=9
after pH=9, solubility increases quickly
reaction H2O + SiO2 <-> HSiO3 + H+ pka =9.8
most natural water’s don’t have pH above 9.8, so doesn’t matter, but if pH is that higher, then solubility also depends on other ions (HSiO3- and H2SiO42-)
explain solubility of SiO2 (quartz) 1st dissociation
SiO2 is an acid, dissociates at high pH first into HSiO3 then to H2SiO42-
reaction: K = H+ * HSiO3- / SiO2(aq) *H2O
increasing pH, H+ does down, SiO2(aq) is constant, so HSiO3- must increase (linearly bc H+ decreases linearly), slope=1
T/F: The solubility of SiO2(aq) is constant with increasing pH
true
explain solubility of SiO2 (quartz) 2nd dissociation
H4SiO4 is an acid, dissociates at high pH first into H3SiO4- then H2SiO42-
reaction: K = H2SiO42- * H+ / HSiO3- * H2O
increasing pH, H going down, HSiO3- is still going up (now in denominator), H2SiO4 must go up 2x, slope=2
what is saturation state?
what is saturation index?
equilibrium of mineral in water
compares ion concentrations with the solubility product
index is log of saturation state
is calcite more or less soluble with increasing pH?
less
explain the states of saturation state
more than 1: supersaturation, system driven towards precipitation
less than 1: undersaturation, system driven towards dissolution
omega: cannot be negative
explain the states of saturation index
more than 0: supersaturation, system driven towards precipitation
less than 0: undersaturation, system driven towards dissolution
details on undersaturated solutions
Q/K = ({Ca2+}*{SiO42-} / K) <1
we need to dissolve more CaSO4 to have enough Ca2+ and SO42- to be in eqlm
need to raise Q to get to eqlm
details on supersaturated solutions
Q/K = ({Ca2+}*{SiO42-} / K) >1
we would need to remove CaSO4 in solution to have less Ca2+ and SO42- from solution to be in eqlm. We have too much Ca2+ and SO42-
T/F: saturation states indicate thermodynamic drive and how quickly it proceeds
false, does indicate thermodynamic drive, but not when the reaction will proceed. that’s kinetics
mystery of dolomite
logQ/K = 3.025, so 10³
it’s very super saturated, but it’s not precipitating in natural environments
rate of reaction is ridiculously low, about 800 years to see 10% growth in dolomite
explain how complexes change solubility
complexation lowers the activity of free ions, and increases the solubility of minerals
CaSO4 is a SOLUTE, not solid
K = CaSO4 / Ca2+ * So42- = 10^(2.5)
perrier water example
3.5 L of CO2 per L H2O
3.5 ×0.043molCO2/LCO2 = 0.1505 mol/L CO2
ambient is 0.8 L CO2 per L H2O
0.8×0.043molCO2/LCO2 = 0.0344 mol/L CO2
3.5/0.8= 4.3. 4 times too much CO2 in perrier than ambient CO2