4.1 - Water Systems (ESS)
1/49
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
50 Terms
Water on Earth composition
Oceans - 96.5%
2.6% - Freshwater:
Glaciers & Ice caps - 80%
Groundwater - 0.6%
Lakes, rivers, soil water, atmospheric water vapor and biota - the rest.
Glaciers & Ice caps - 1.7%
Groundwater - 1.7%
Surface freshwater - 0.2%
atmosphere - 0.001%
organisms - 0.0001%
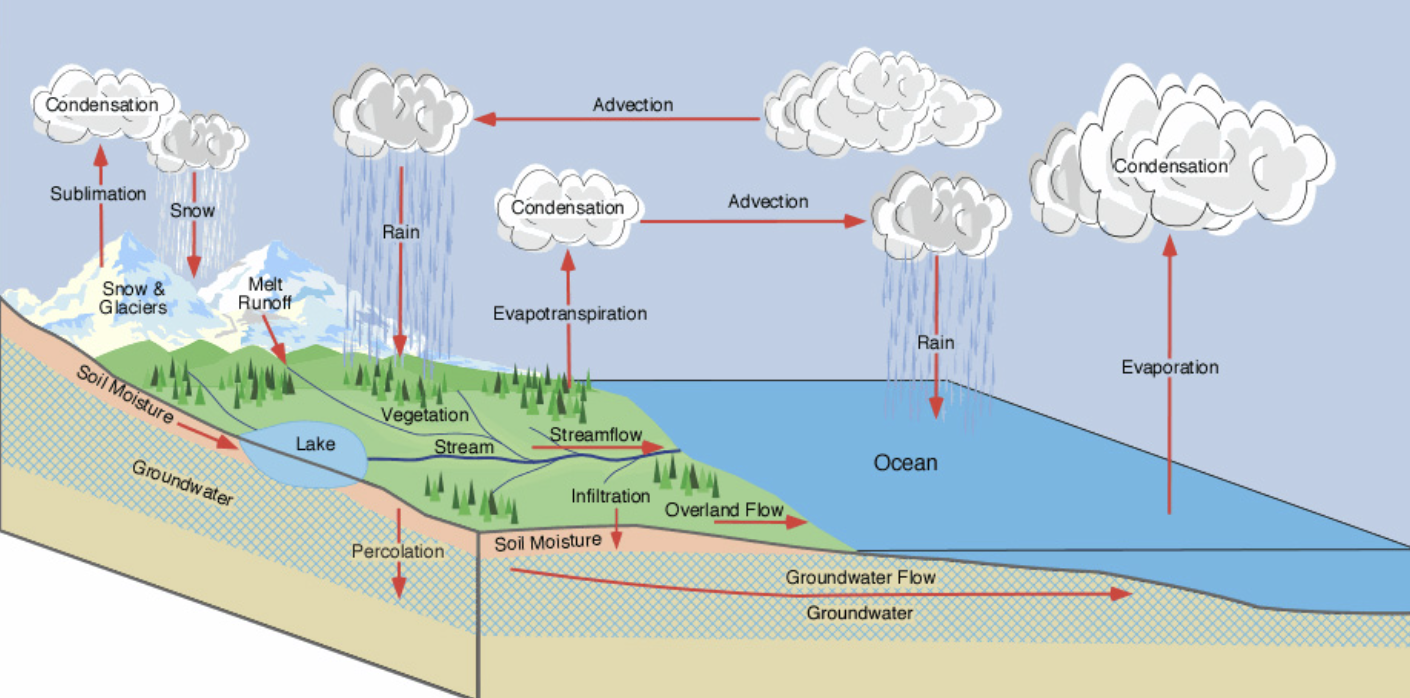
Hydrological cycle
Shows how water moves between different stores. These flows are driven by energy from solar radiation and gravity.
Evaporation
Energy from the sun heats up water so it evaporates (transformation - from water to gas). Water requires a high amount of energy to heat up. Evaporating water - used to cool down bodies and buildings.
Transpiration / evapotranspiration
The psychological loss of water in the form of water vapor, mainly from the stomata in leaves, but also through evaporation from the surface of leaves, flowers, and stems. It is the movement of water around the plant.
Condensation
The transformation when water turns back to a liquid state and releases heat back into a system. Water is condensed and stored in clouds. Releases latent heat energy, which is crucial for regulating Earth’s energy balance and drives weather patterns.
Advection
Transfer - the movement of clouds in the atmosphere by winds.
Precipitation
Transfer - after the water is condensed, it then can fall back from the sky as precipitation. It returns in the form of rain, now, sleet, or hail.
Freezing
Transformation - water turns into a solid state.
Melting
Transformation - solid state water turns into liquid state.
Sublimation
Transformation - Water molecules move directly from the solid state to the gaseous state.
Streamflow
Water moves from watersheds (the point where rivers start) at higher altitudes to the oceans and seas that are at a lower altitude.
Infiltration
Water enters the soil and replenishes groundwater stores.
Percolation
movement and filtering of water through porous materials (rocks and soil).
Surface runoff
The flow of water over the surface.
Waterlogged soil
When soil reaches it’s capacity, because it is too compacted, or precipitation is so heavy that the water is arriving faster than it can infiltrate the ground.
Steady state
inputs = outputs
Stores of the cycle
Oceans
Ice caps and glaciers (polar regions)
Groundwater (in aquifers)
Lakes and rivers
Atmosphere (clouds and water vapor)
Soil Moisture (stored within the soil)
Flows
Evaporation
Precipitation
Runoff
Infiltration
Transpiration
Percolation
Water Budget
A quantitative estimate of the amount of water in stores and flows of the water cycle.
Land Cover & Urbanization
Land cover can slow or speed up the flow of water as surface runoff or as infiltration. Urbanization tends to increase the amount of land covered by cement and asphalt/tarmac. When water falls on these surfaces it quickly runs off, straight into storm drains or water courses. This can also carry sediment and minerals. Forests tend to slow down the surface runoff and increase infiltration, so deforestation, conversely, increases these factors. The local government are also in the process of increasing the size of the storm drains to cope with the increased intensity of rainfall events which were causing flooding.
Farming & agriculture
Farming practices (agriculture) impact the land cover and soil characteristics. Leaving soil bare tends to increase surface runoff and loss of top soil. Using heavy machinery on land can lead to compaction of the soil. This means that the soil pore spaces are smaller and so water doesn't infiltrate as quickly. This then increases surface runoff. Farming practices can be adapted to improve soil infiltration and reduce problems of runoff. Both the increased coverage of land by impermeable surfaces through urbanisation and farming practices which compact soil can lead to an increased risk of flooding, even flash floods when the water is not able to infiltrate the soil quickly enough.
Deforestation and Afforestation
Trees and shrubs result in a slowing of water as it reaches the ground. This is called interception - the leaves intercept the water droplets. This protects the soil and it reduces surface runoff but it also means the water is more likely to infiltrate the soil. Soils with trees on them are also more spongy as they contain more air spaces. Another nature-based solution to adapt to climate change is to design spongy cities.
Forests - Hydrological cycle
Forests play an important role in providing rain for locations far from oceans.
Amazon forest:
Trees transpire around from 200 to 1000 liters of water per day
Huge jets of rapid, humid air that constantly flows above the canopy - ‘Flying rivers’ carry about 20 billion tons of water through the air.
Equator → trade winds blow from east to west which also blow the flying rivers in the same direction before encountering the Andes
The Andes mountains act as a giant barrier, causing the winds and rivers to redirect southwards.
When flying rivers meet cold air they grow heavier and release torrents of water. In this way they bring rain, cooler temperatures, and humidity to much of South America.
Clearing the amazon for agriculture and industry is causing flying rivers to dry up, leading to drought and hotter temperatures across South America.
If this continues, swaths of the continent may be reduced to desert in a matter of decades.
The northwest of the Peruvian Amazon is the territory of the Wampis Nation, a community of over 15,000 people who manage over 130,000 square km of land.
Indigenous community that lived in the rainforest for thousands of years, practicing sustainable hunting, fishing, and agriculture.
They are battling extractive industries - and policies that sanction them.
Since 1960s the Peruvian government has been licensing the Wampis’ territory to corporations for gold mining and oil extraction - threaten life of ecosystem.
2015: the community formed the Autonomous Territorial Government that seeks recognition as a government responsible for their own lands, forests, and internal affairs.
In its policies the Wampis nation prioritizes collective land ownership, cultural preservation, and conservation of plants and animals and natural cycles that protect the rainforest.
Living in harmony with nature.
They monitor the wind, measure rainfall and weigh water levels in leaves and soil.
2016-2018: they protested against illegal gold mining along the Santiago river, uncovered mercury pollution, guarded the area, and attacked illegal machinery for months, eventually expelling the miners. In 2017 Wampis successfully petitioned a court to bar a private oil company from their land.
Water compound
composed of one oxygen atom and two hydrogen atoms joined together by covalent bonds - H2O
Oxygen atom
large atom compared to hydrogen. It contains eight electrons, six of which are located in its outer shell. It is also one of the most electronegative atoms and so it has a tendency to attract shared electrons towards itself.
Hydrogen atom
smallest element and has only one electron in total.
Universal solvent
Water is a highly polar molecule with an uneven distribution of charge. This results in water being a universal solvent. Any salts and any polar compound will dissolve in water. Once dissolved, some substances then react with water. Blood is largely water and gases and salts are dissolved in the water component.
Cohesive properties
water molecules stick to each other giving water a surface tension (pond skaters exploit this property).
Adhesive properties
water sticks to other substances.
Capillary action in plants
Water molecules move up the xylem in plant stems by capillary action - the adhesive properties mean the water is sticking to the sides of the xylem - while the cohesive properties mean that the water molecules stick to each other and can be carried up tens of metres to the top of a tree simply by the evaporation of water from the stomata in the leaves
Crystalline structure
a lattice - that is regular in shape and means the molecules are more widely spaced (resulting in lower density) than in the liquid state. This leads to ice floating on water and taking up more space than in the liquid state (a highly unusual property). This allows life to survive during winters when the ice forms on the surface of water bodies and actually insulates the water below (if the water body is deep enough).
Hydrogen - heat capacity
it takes a lot of energy to raise the temperature of water and for water to change state from solid to liquid to gas. It means that a lot of energy is released in the opposite direction when water condenses or freezes. This means that water has a high specific heat capacity and high latent heat of vapourisation. This leads to the milder climates found in coastal regions and the cooling properties of water evaporating.
Warming water
Both oxygen and carbon dioxide increase in (gas) solubility as the temperature of water decreases. Therefore warming water means less oxygen and carbon dioxide for water living organisms. As we will learn soon, water bodies are important carbon sinks but as waters warm, less carbon dioxide is able to be held in the water. This is a positive feedback loop. As pressure increases, gas solubility increases in water. Thus, in deeper water there are higher concentrations of dissolved gases due to higher pressure and colder temperatures.
Cold water
Denser than hot water as molecules have less energy and therefore take up less space than warmer water. More molecules per unit area means a greater density. Cold water sinks and hot water rises.
Saline water
Heavier and more dense than freshwater, it therefore sinks.
Transparency of water
Depends on how much particulate matter is in the water or how turbulent the water is.
Particulate matter
can be inorganic such as sediment from erosion or organic such as plankton or algae.
Turbulence
increases the formation of oxygen bubbles which decreases the transparency.
As marine organisms die or excrete waste…
… much of this falls to the bottom of the oceans and this sediment can either become future limestone (inorganic carbonates) (calcium carbonate) or future fossil fuels (carbon compounds in organic matter that does not fully decompose).
Phytoplankton photosynthesis
Carbon dioxide dissolves in water and the action of phytoplankton photosynthesis has helped turn about one third of the rising carbon dioxide levels in the atmosphere into sequestered carbon. It is not possible for oceans to keep on absorbing more carbon dioxide.
Reaction
Carbon dioxide (CO2)reacts with water to form carbonic acid (H2CO3) and this dissociates into hydrogen ions (H+) hydrogen bicarbonate (HCO3-)and carbonate ions(CO32-) (the same process happens in your blood and is used to regulate your blood pH).
effect of the reaction of carbon dioxide dissolving in water
There is a dynamic equilibrium in this dissociation but a decrease in pH (an increase in H+) pushes the equilibrium to the left of the equation and means that the carbonate ions are less available for marine creatures that make shells or carbonate based skeleton structures. These include corals, clams, some phytoplankton and other shelled organisms.
Thermocline
a band of rapidly declining water between the two layers.
Colder deeper water has?
more oxygen and nutrients, and so the availability of these in the water body will depend on stratification of the water.
Stratification
The layers if water in water bodies.
Impact of climate change on stratification
the ocean layers(the stratification) are intensifying and becoming more stable. This is strongest in the upper 200 metres of water. This means that currents that would normally bring nutrients up from the ocean floor (upwelling) are becoming less strong. This will reduce productivity in coastal regions.
The thermo-haline circulation is slowing down due to
Melting of sea ice (ice is pure water and does not contain salt) means freshwater is entering the arctic and antarctic oceans. This is reducing salinity in these most northerly and southerly parts of our oceans. Normally these areas are our most saline areas and the heavy dense water sinks at these locations to start the thermohaline circulation.
Gyres
large surface currents - pushed by winds that are the result of atmospheric pressure differences and the spinning of the Earth around its axis, known as the coriolis effect. These big surface currents can impact the climate of the land that they interact with, for example, the warm water current, the Gulf Stream / North Atlantic Drift keeps North-West Europe milder than it should be for its latitude. The cold water current, the Benguela current brings cold mists to the Namibian coast of southern Africa.
Upwellings
occur in a few places around the world where the land heats up and causes wind to blow off the land over the coastal waters, pushing this coastal water away from the coast. If there is deep ocean near the coast, then this water is replaced by nutrient rich water from the deep oceans (all those fallen ocean sediments). This leads to a very productive coastal marine fishery ecosystem in a few places around the world, such as Peru.
ENSO effect on the thermocline
Normally water is warmer over the Western Pacific (Oceania and Indonesia) and trade winds push water towards the west. This results in the thermocline between warmer surface water and deeper colder water being deeper in the west and shallower in the east. This means that the nutrient rich waters are more easily brought to the surface in the eastern Pacific around the west coast of South America. ENSO events slow these trade winds and mean that warm water starts accumulating in the eastern Pacific, pushing this thermocline deeper and slowing the upwelling of nutrient rich waters that leads to a decline in the fisheries there.